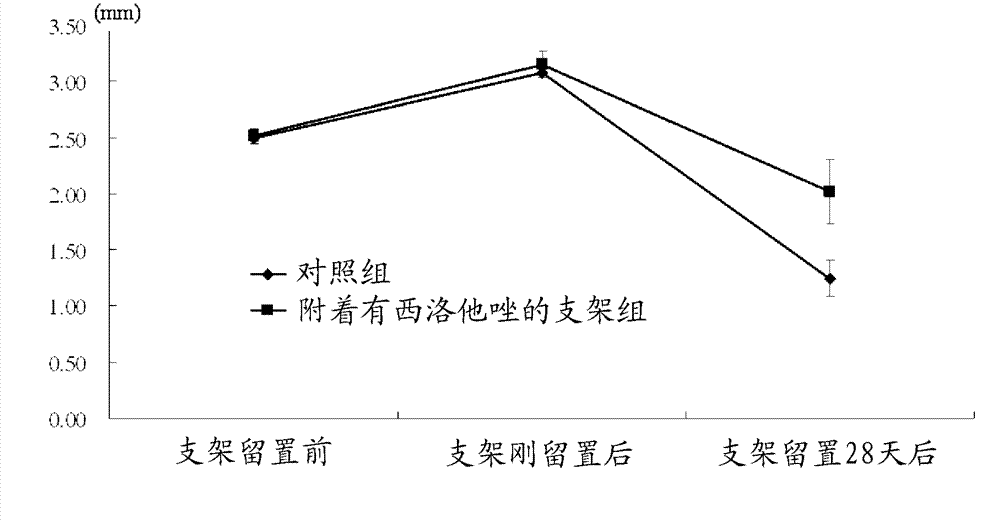
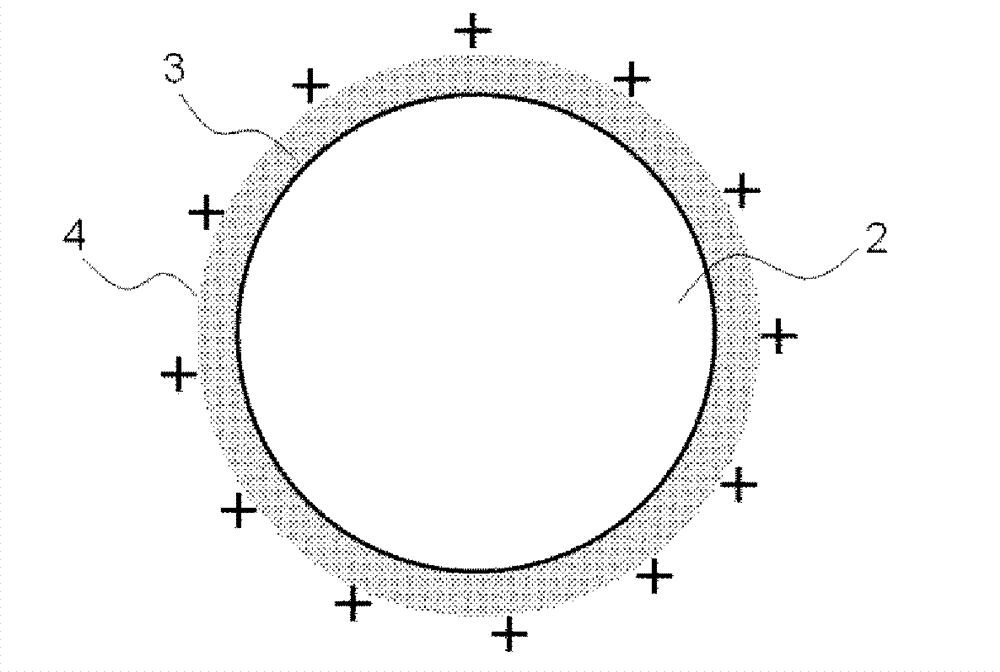
Medical device for placement into a lumen and manufacturing method thereof
A technology for medical equipment and manufacturing methods, applied in pharmaceutical formulations, catheters, pharmaceutical sciences, etc., can solve problems such as inability to obtain sufficient drug effects, solvent limitation, and lack of versatility in coating technology, and achieve improved efficiency and improved intake. , the effect of increased cell adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1 (Preparation 1 of Drug Particle Suspension)
[0176] A solution was prepared by dissolving 1 g of probucol in a mixed solution of 40 mL of acetone and 20 mL of ethanol as a good solvent. In addition, 15 g of an aqueous solution of 2% by weight of chitosan (CHITOSAN GH-400EF, manufactured by NOF) was mixed with 100 mL of an aqueous solution of 2% by weight of polyvinyl alcohol (Gosenol EG05, manufactured by Nippon Synthetic Chemical Industry Co., Ltd.) to prepare a solution relative to probucol solutions in poor solvents. In this solution, under stirring at 40°C and 400rpm, the previous probucol solution was added dropwise at a certain speed (20mL / min), and the crystalline drug particles (probucol) were obtained through the diffusion of the so-called good solvent into the poor solvent. test) suspension.
[0177] Next, acetone and ethanol were distilled off under reduced pressure. The zeta potential on the particle surface was measured by electrophoresis (M...
Embodiment 2
[0183] Example 2 (Preparation 2 of Drug Particle Suspension)
[0184] The drug particles were prepared under the same conditions as in the manufacturing method of Example 1 except that the drug particles were not pulverized by a desktop ball mill, and the average particle diameter of the drug particles was 21.5 μm.
Embodiment 3
[0185] Example 3 (Preparation 3 of Drug Particle Suspension)
[0186] Drug particles were prepared under the same conditions as in the production method of Example 1 except that 60 g of the 2% by weight chitosan aqueous solution added to the solution corresponding to the poor solvent was used. The average particle diameter of the drug particles by the laser diffraction scattering method was 4.5 μm. The ξ potential was +45mV, the content rate of probucol in the drug particles was 53%, and the pass rate was 65%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com