Immiscible water/polymer two-phase electrolyte and battery
A hydrophobic polymer and electrolyte technology, applied in secondary batteries, fuel cell-type half-cells, and secondary battery-type half-cells, circuits, etc., to achieve high power density, good contact, and high energy density.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A lithium-air battery, the battery is composed of an electrolyte, a positive electrode, a negative electrode and a wire; wherein, the electrolyte is an immiscible "water / polymer" two-phase electrolyte, composed of an aqueous electrolyte and a hydrophobic organic polymer The composition of the electrolyte, the aqueous electrolyte and the hydrophobic organic polymer electrolyte are incompatible with each other and form a stable and clear interface; among them, the aqueous electrolyte is used for the positive electrode, and the hydrophobic organic polymer electrolyte is used for the negative electrode; the positive electrode is air The electrode is located in the aqueous electrolyte; the negative electrode is a metal lithium electrode, embedded in a hydrophobic organic polymer electrolyte; the external circuit between the positive electrode and the negative electrode is connected with the load (electrical appliance) by a wire, the wire is wrapped with an insulating material, ...
Embodiment 2
[0044] According to the method described in Example 1, the hydrophobic organic polymer electrolytes containing different mass percentages of polymethyl methacrylate were prepared; wherein the concentration of lithium perchlorate was 0.5 mol / L, and the hydrophobic organic The mass of the polymer electrolyte is 100%, and the mass percentages of polymethyl methacrylate are 0%, 5%, 10%, 15%, 20%, 25% and 30% respectively.
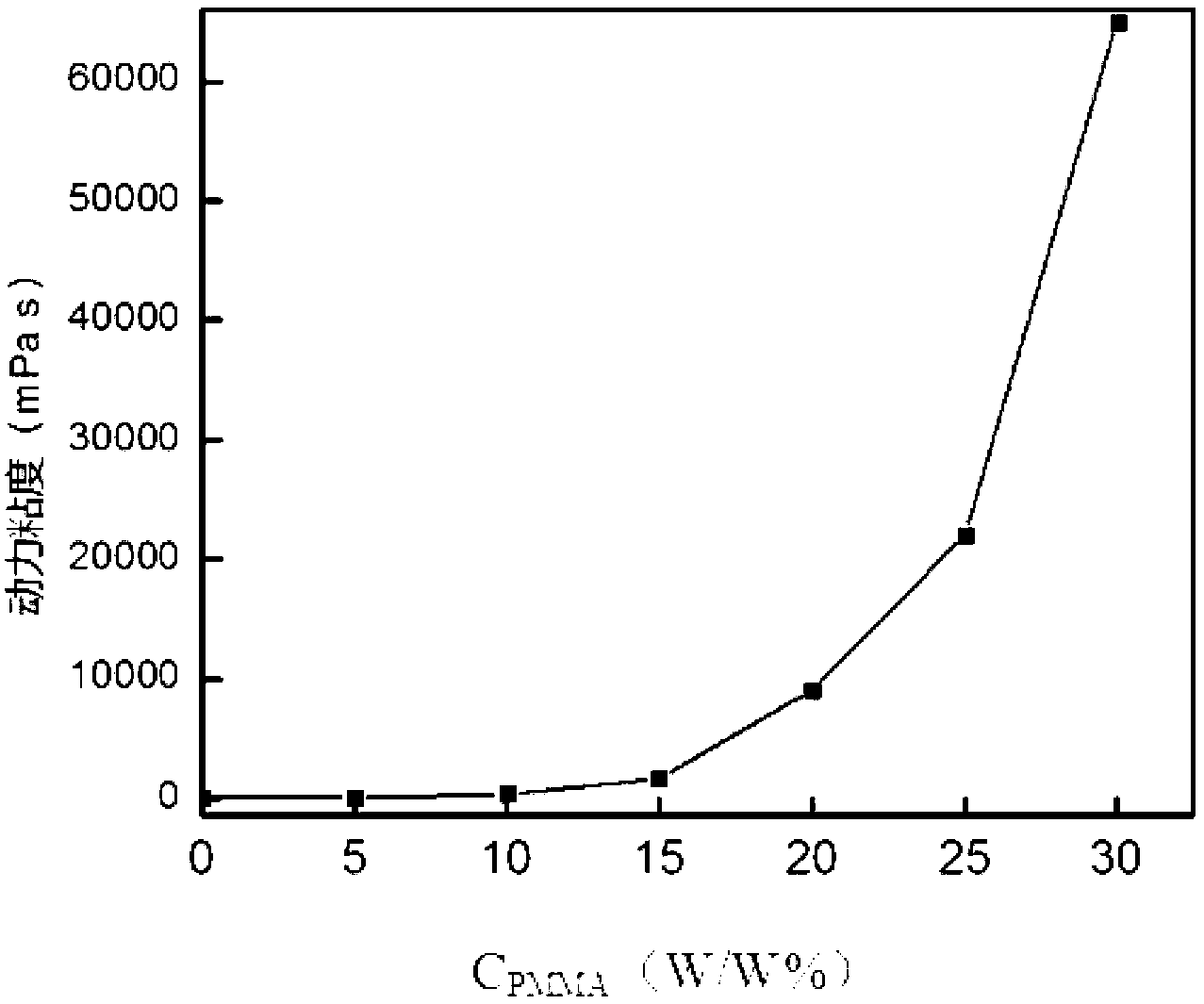
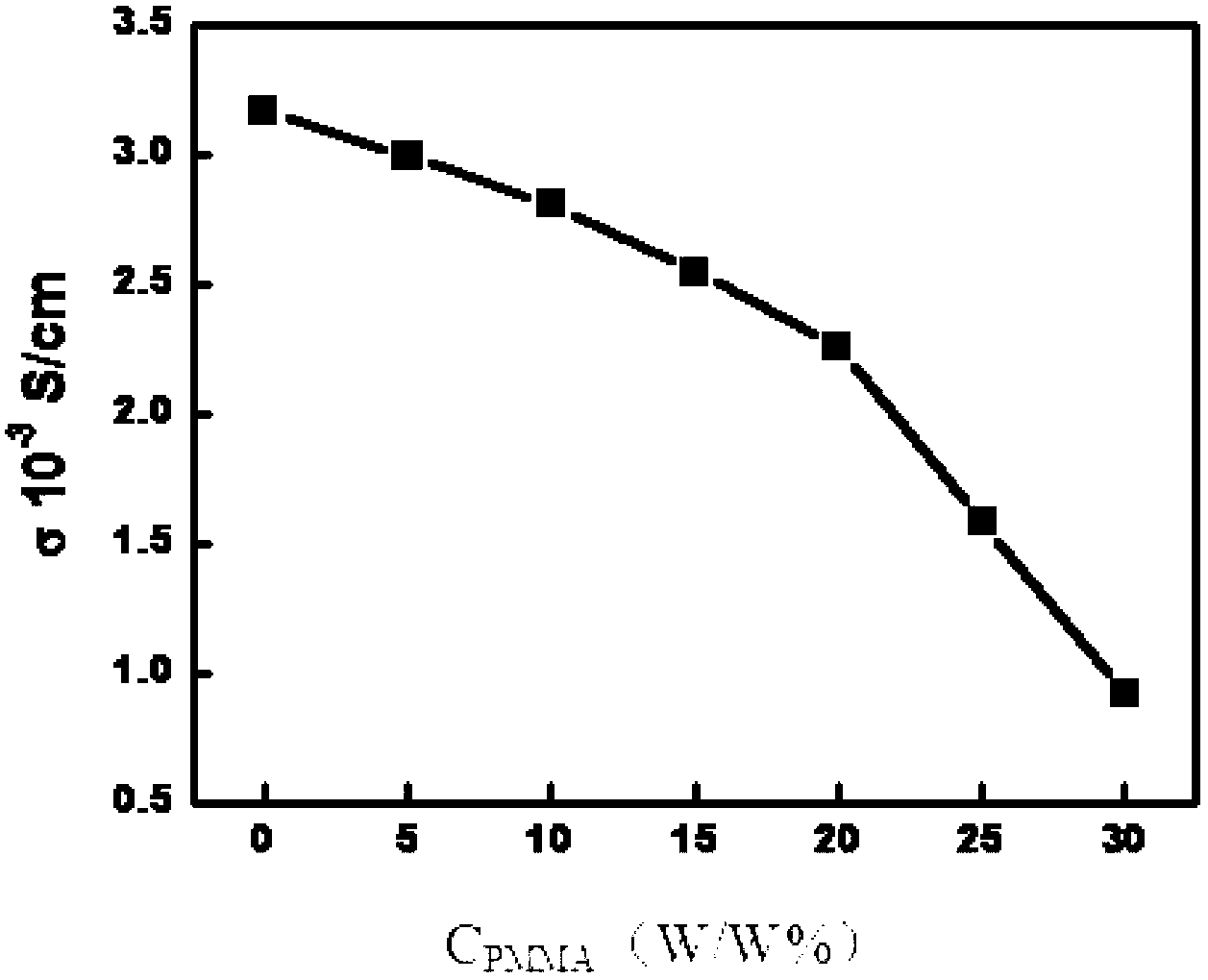
[0045] The viscosity (σ) and conductivity (η) of the hydrophobic organic polymer electrolyte were measured by AC impedance technology, and the measurement was performed on the CHI660A electrochemical workstation (Shanghai Chenhua Company) using double platinum conductivity electrodes, and the results were as follows figure 1 with figure 2 Shown.
[0046] figure 1 The horizontal axis in is the mass percentage concentration of polymethyl methacrylate (PMMA) in the hydrophobic organic polymer electrolyte, expressed as C PMMA , The unit is W / W%, the vertical axis is t...
Embodiment 3
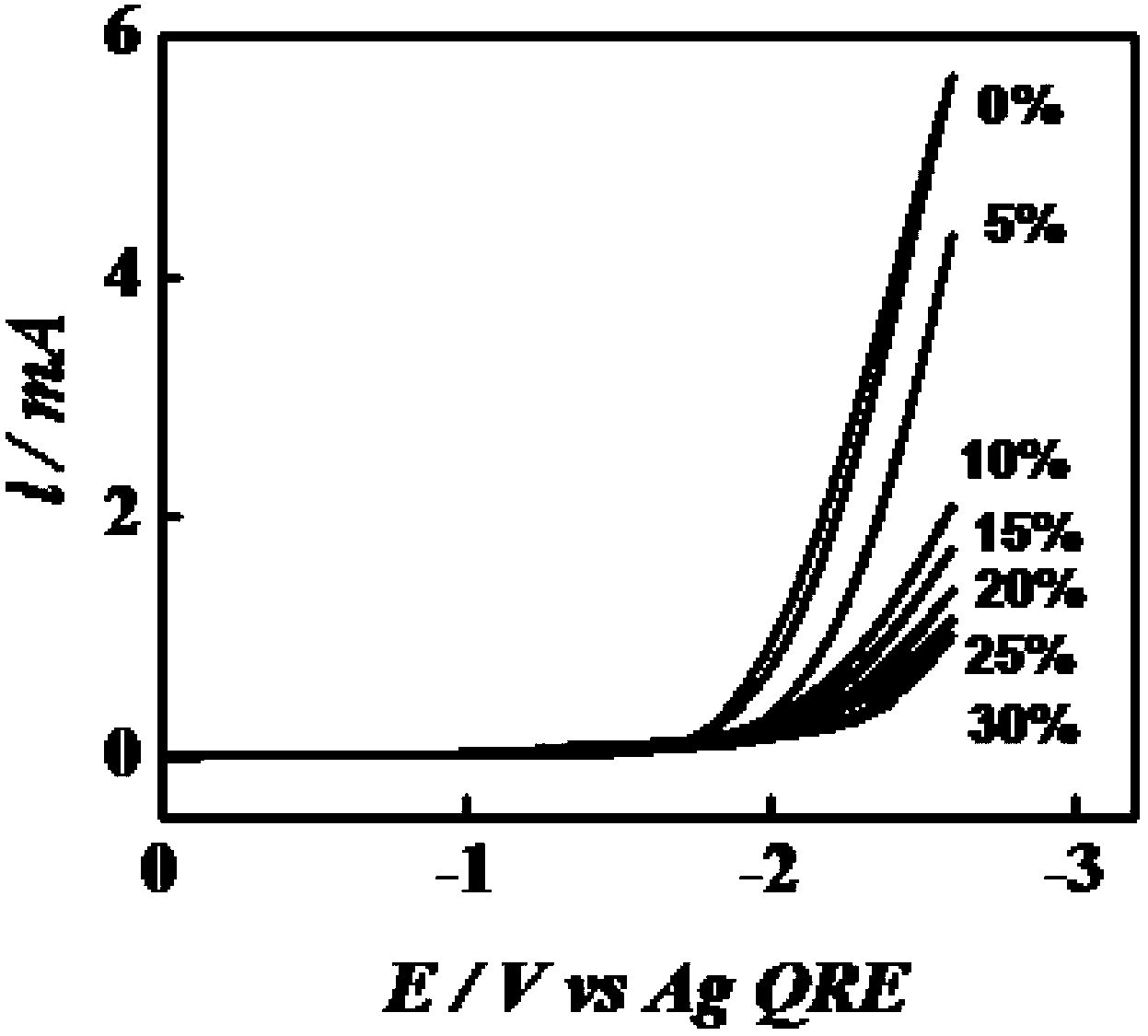
[0048] A three-electrode system was used to measure the hydrophobicity and oxygen barrier properties of the hydrophobic organic polymer electrolyte prepared in Example 2 respectively. The three-electrode system is prepared by the following method:
[0049] The hydrophobic organic polymer electrolyte is divided into two groups. One group is used to measure the hydrophobicity, which is saturated with water vapor at 22±2℃ and then transferred to a glass electrolytic cell. The other group is used to measure the oxygen barrier performance. After being saturated with oxygen at ±2℃, it is transferred to a glass electrolytic cell. In each electrolytic cell, a platinum ultramicrodisk electrode with a diameter of 25 microns is used as the working electrode, and a silver (Ag) wire with a diameter of 0.5 mm is used as the quasi-reference electrode. The Ti electrode with a diameter of 0.5 mm is used as the counter electrode. The three electrodes are placed in the hydrophobic organic polymer e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com