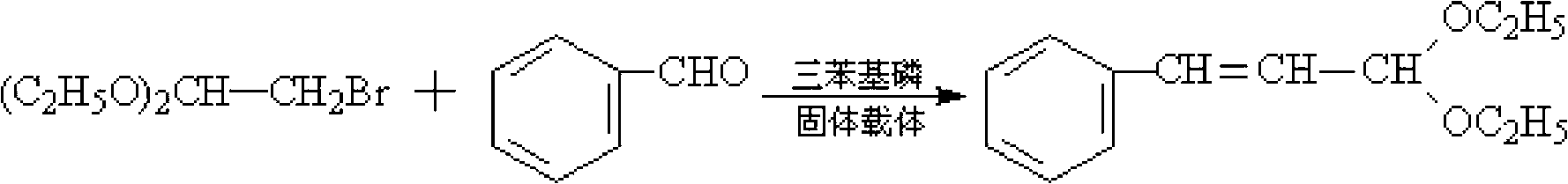
Preparation method of cinnamaldehyde diethylacetal
A technology of cinnamaldehyde diethyl acetal and diethoxyethane, which is applied in the field of preparation of cinnamaldehyde diethyl acetal, can solve the problems of unsuitability for industrial production, low yield, easy hydrolysis, etc., and achieve easy removal , cost reduction, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 10g of triphenylphosphine, 101.5g of toluene, 0.4mol of benzaldehyde (M=106) and 0.3mol of 2-bromo-1,1-diethoxyethane (M=197) into a flat-bottomed flask and mix thoroughly Then pour it into a tubular reactor equipped with a macroporous resin carrier loaded with NaOH, put it into a microwave oven, and install a thermometer and a condenser. Microwave irradiation was carried out under the state of stirring and normal pressure, the power was 600W, the temperature was raised to 85°C, and the temperature was kept at reflux for 1.5h. After the reaction is completed, cool to room temperature after the reaction to obtain a reaction liquid, then distill off the solvent toluene under normal pressure, filter out the catalyst triphenylphosphine, and then further distill the reaction liquid through a scraper type molecular distillation device to obtain the product cinnamaldehyde diethyl acetal. The purity of the product is 99%, and the yield is 92.8%.
example 2
[0022] Add 10g of triphenylphosphine, 111g of toluene, 0.4mol of benzaldehyde and 0.35mol of 2-bromo-1,1-diethoxyethane into a flat-bottomed flask, mix well and pour into a macroporous resin loaded with KOH Carrier tube reactor, put it into a microwave oven, and install a thermometer and a condenser. Microwave irradiation was carried out under the state of stirring and normal pressure, the power was 550W, the temperature was raised to 88°C, and the temperature was kept at reflux for 1.5h. After the reaction is completed, cool to room temperature after the reaction to obtain a reaction liquid, then distill off the solvent toluene under normal pressure, filter out the catalyst triphenylphosphine, and then further distill the reaction liquid through a scraper type molecular distillation device to obtain the product cinnamaldehyde diethyl acetal. The purity of the product is 99%, and the yield is 93.3%.
example 3
[0024] Add 10 g of triphenylphosphine, 117 g of toluene, 0.4 mol of benzaldehyde and 0.38 mol of 2-bromo-1,1-diethoxyethane into a flat-bottomed flask, mix well, and pour into a loaded K 2 CO 3 Put it into a tubular reactor with a macroporous resin carrier, put it in a microwave oven, and install a thermometer and a condenser. Microwave irradiation was carried out under the state of stirring and normal pressure, the power was 500W, the temperature was raised to 90°C, and the temperature was kept at reflux for 2h. After the reaction is completed, cool to room temperature after the reaction to obtain a reaction liquid, then distill off the solvent toluene under normal pressure, filter out the catalyst triphenylphosphine, and then further distill the reaction liquid through a scraper type molecular distillation device to obtain the product cinnamaldehyde diethyl acetal. The product purity is 99%, and the yield is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com