Lywallzyme of phage of staphylococcus aureus as well as preparation method and application thereof
A technology of staphylococcus aureus and staphylococcus, applied in the field of bioengineering, can solve problems such as multi-drug resistance, difficult clinical treatment, and drug resistance of staphylococcus aureus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Phage Isolation Preparation and Genome Extraction
[0046] Phage isolation and preparation
[0047] The sample of the present invention is collected from the milk sample of Xigang Dairy Farm in Nanjing, Jiangsu. In the milk sample, add 1ml of Staphylococcus aureus overnight culture, then add sterile CaCl 2 After mixing the mother liquor to a final concentration of 1.25mM, add 20ml TSB-YE medium, let it react at room temperature for 30min, and then place it at 30°C. -1 , 4°C, centrifuge for 30 min, take the supernatant; then filter the supernatant with a 0.22 μm filter membrane to form a phage stock solution.
[0048] Take 0.1ml of bacteriophage stock solution, carry out 10-fold dilution, take 10 2 、10 4 and 10 6 Mix 0.1ml each of the dilution solution with 0.1ml of the overnight cultured host bacterial solution. After 15 minutes at room temperature, add about 5ml of 0.7% LB medium. It was solidified, placed in an incubator at 30° C. for 12 hours, and then...
Embodiment 2
[0052] Embodiment 2: LysM9 gene ( lys M9) cloning and expression vector construction
[0053] a. Design a pair of specific primers according to the LysM9 gene coding sequence:
[0054]M9-1: 5’- ATT GGA TCC GGT AGG TGT TGA CCA ATG TTG -3', see SEQ ID NO.3;
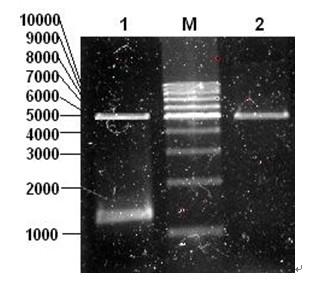
[0055] M9-2: 5’-CTT AAG CTT TAA ATC GTG CTA AAC TTA CC -3', see SEQ ID NO.4; use the phage genome as a template to amplify the full-length sequence of the LysM9 gene with the above-mentioned specific primers, electrophoresis on 1% agarose, and identify the size of the amplified fragment;
[0056] b. Gel cutting recovery (1.5kb fragment) to amplify the correct target fragment, the gel recovery kit will purify and recover the fragment, and connect the fragment to the pMD18-T vector overnight at 16°C, and transform Escherichia coli DH5α competent cells the next day , smeared on a plate containing ampicillin (100 μg / ml), and sequenced and identified the transformed positive clone, named it LysM9 gene, and its nucleotide...
Embodiment 3
[0061] Example 3: Induced expression and purification of LysM9 protein
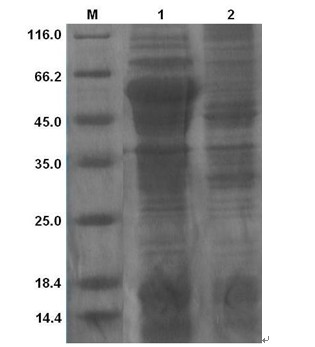
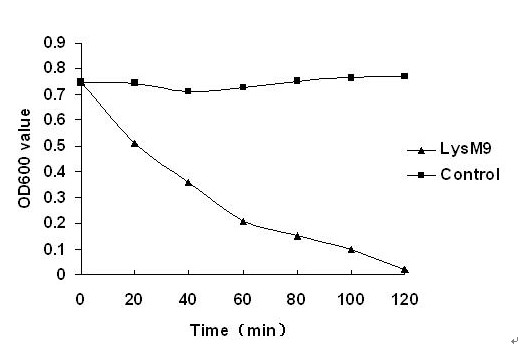
[0062] The recombinant strain DE3 (pET-lysM9) was inoculated into LB culture medium containing kanapenicillin (100 μg / mL), and cultured overnight at 37°C with shaking; the next day, it was transferred to 100 mL LB medium at a ratio of 1:100, Shake culture at 37℃ to OD 600 When the value is about 0.5, add IPTG to a final concentration of 1 mmol / L, and induce at 28°C for 5 h. Collect the bacteria, disrupt the cells by ultrasonic, centrifuge at 10,000 rpm / min at 4°C for 10 min, collect the supernatant, filter the supernatant through a 0.22 μm filter membrane, and analyze the protein expression in the lysed supernatant by SDS-PAGE. The filtered lysed supernatant was purified by His affinity chromatography nickel column (GE Healthcare, Sweden), specifically according to the instructions of the kit. The obtained protein was named lysM9, and the purified lysM9 product was detoxified (≤0.01 EU / μg endotoxin) wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com