Preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide
A technology of amino alkyl sulfonamide and amino alkyl sulfonyl chloride, which is applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve the problems of troublesome post-processing, poor reproducibility, low yield, etc., and achieves high biological activity and operation Simple, reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
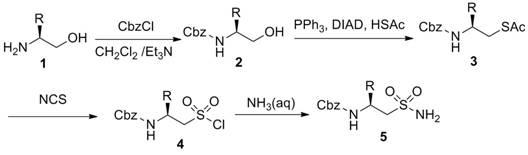
[0031] The present invention provides a N -The preparation method of benzyloxycarbonyl-2-aminoalkylsulfonamide, the steps comprise:
[0032] S1: using o-amino alcohol represented by formula [1] as raw material, reacting with benzyl chloroformate to obtain N - benzyloxycarbonyl-2-aminoalcohol;
[0033] S2: Take the result of step S1 N - Benzyloxycarbonyl-2-aminoalcohol and thioacetic acid undergo Mitsunobu reaction (Mitsunobu) to obtain the acetate of aminothiol;
[0034] S3: use the acetate of aminothiol obtained in step S2 N -Oxidation of chlorosuccinimide to N - benzyloxycarbonyl-2-aminoalkylsulfonyl chloride;
[0035] S4: the obtained step S3 N -benzyloxycarbonyl-2-aminoalkylsulfonyl chloride is obtained through ammonolysis shown in formula [5] N - benzyloxycarbonyl-2-aminoalkylsulfonamide;
[0036] Formula 1] Formula [5];
[0037] Wherein, the R is selected from one of hydrogen, alkyl, aryl, aralkyl, alkoxyalkyl, alkylaminoalkyl, and aryloxya...
Embodiment 1
[0060] (1) Dissolve 50 mmol of ethanolamine as o-amino alcohol in 165 mL CH 2 Cl 2 in, at 0 o Under C conditions, 8.6 mL (10.2 g, 60 mmol) of benzyl chloroformate was added dropwise to the system, followed by 35 mL (25.3 g, 250 mmol) of Et 3 N, stirred overnight at room temperature, stopped the reaction, removed the solvent under reduced pressure to obtain an oil, separated and purified by silica gel column chromatography to obtain N - O-aminoalcohol protected by benzyloxycarbonyl.
[0061] (2) at -10 o Under C conditions, the Ph 3 P (10.48 g, 40 mmol) was dissolved in 48 mL of anhydrous THF, and it was slowly added dropwise (over 30 min) to 24 mL containing diisopropyl azodicarboxylate (DIAD) (8.0 g, 40 mmol ) in anhydrous THF solution, and then at -10 o Under the condition of C, react for 0.5 h, and a white precipitate will form during the reaction. will subsequently N - 48 mL of anhydrous THF solution of benzyloxycarbonyl-protected o-amino alcohol (20 mmol) and HSAc...
Embodiment 2
[0065] ( S The preparation of )-2-benzyloxycarbonylaminopropyl sulfonamide 5b
[0066] Adopt the method identical with embodiment 1 to prepare ( S )-2-Benzyloxycarbonylaminopropylsulfonamide 5b , the difference is that the o-amino alcohol used in the step (1) is (S)-alaninol, and a colorless needle-like solid is obtained with a yield of 63% and a melting point of 150-151 o C, [α] 20 D = +10.8 (c, 1.0, MeOH).IR v (cm-1): 1668 (C=O), 1342 & 1142 (SO 2 ); 1 H NMR (300 MHz, DMSO-d6) δ : 1.24 (d, J = 6.6 Hz, 3H, CH 3 ), 3.04 (dd, J = 7.5, 13.9 Hz, 1H in CH 2 SO 2 ), 3.25 (dd, J = 5.3, 13.9 Hz, 1H in CH 2 SO 2 ), 4.01( ddq, J = 5.3, 13.9, 7.5 Hz, 1H, CHN), 5.03 (s, 2H, OCH 2 ), 6.90 (s, 2H, NH 2 ), 7.28-7.42 (m, 6H, ArH & NH); 13 C NMR (75 MHz, DMSO-d6) d : 20.4, 43.2, 59.8, 65.3, 127.7, 127.8, 128.3, 137.0, 155.2; HRMS (ESI) Calcd. for C 11 h 17 N 2 o 4 S [M+H]+ m / z 273.0904; Found 273.0902.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com