Quality detecting method for traditional Chinese medicine Qianliening preparation
A technology for traditional Chinese medicines and preparations, which is applied in the field of quality inspection of traditional Chinese medicine Qianlenin preparations, can solve the problems that the quality of this product cannot be fully and accurately controlled, the quality inspection method of Qianlenin capsules is not found, and the quality standards need to be improved, so as to achieve enhanced product quality. Controllability, saving analysis time, and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1: the identification method of Chinese medicine Qianlening Capsules
[0146] A. Identification of Myrobalan
[0147] Take 5g of the content of the Chinese medicine Qianlening capsule, add 25ml of absolute ethanol, ultrasonically treat for 30min, add 2g of diatomaceous earth, mix well, filter, and the filtrate is concentrated to 2ml, as the test solution; another 1g of myrobalan reference drug, Add 25ml of absolute ethanol, sonicate for 30min, add 2g of diatomaceous earth, mix well, filter, and concentrate the filtrate to 2ml as the reference drug solution; take another gallic acid reference substance, add methanol to make a solution containing 1mg per 1ml, As a reference solution; test according to thin-layer chromatography (Appendix VI B of "The Pharmacopoeia of the People's Republic of China" 2010 Edition), draw 5 μl of each of the above solutions, and place them on the same silica gel G thin-layer plate respectively. 6:4:1 chloroform-ethyl acetate-formic...
Embodiment 2
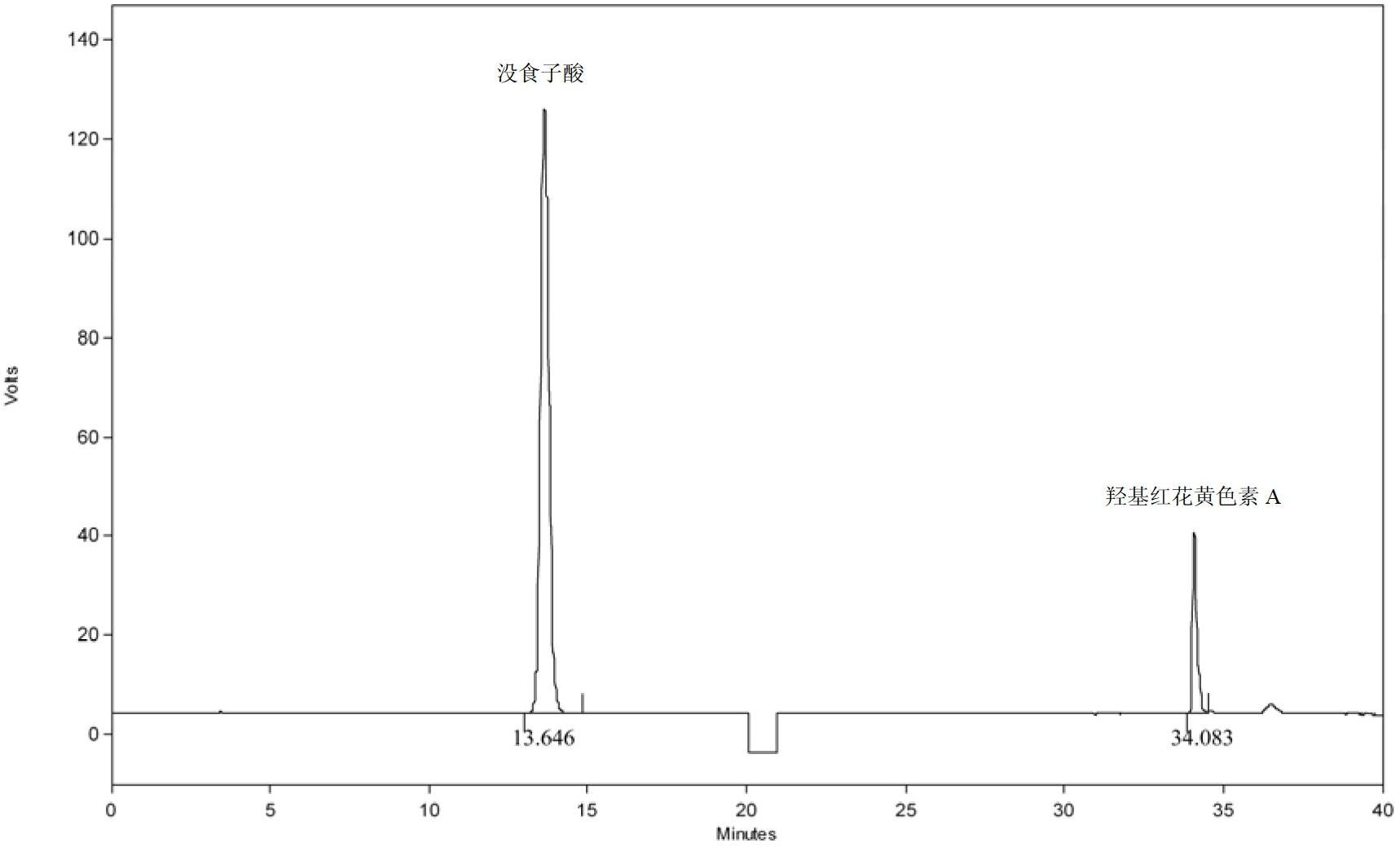
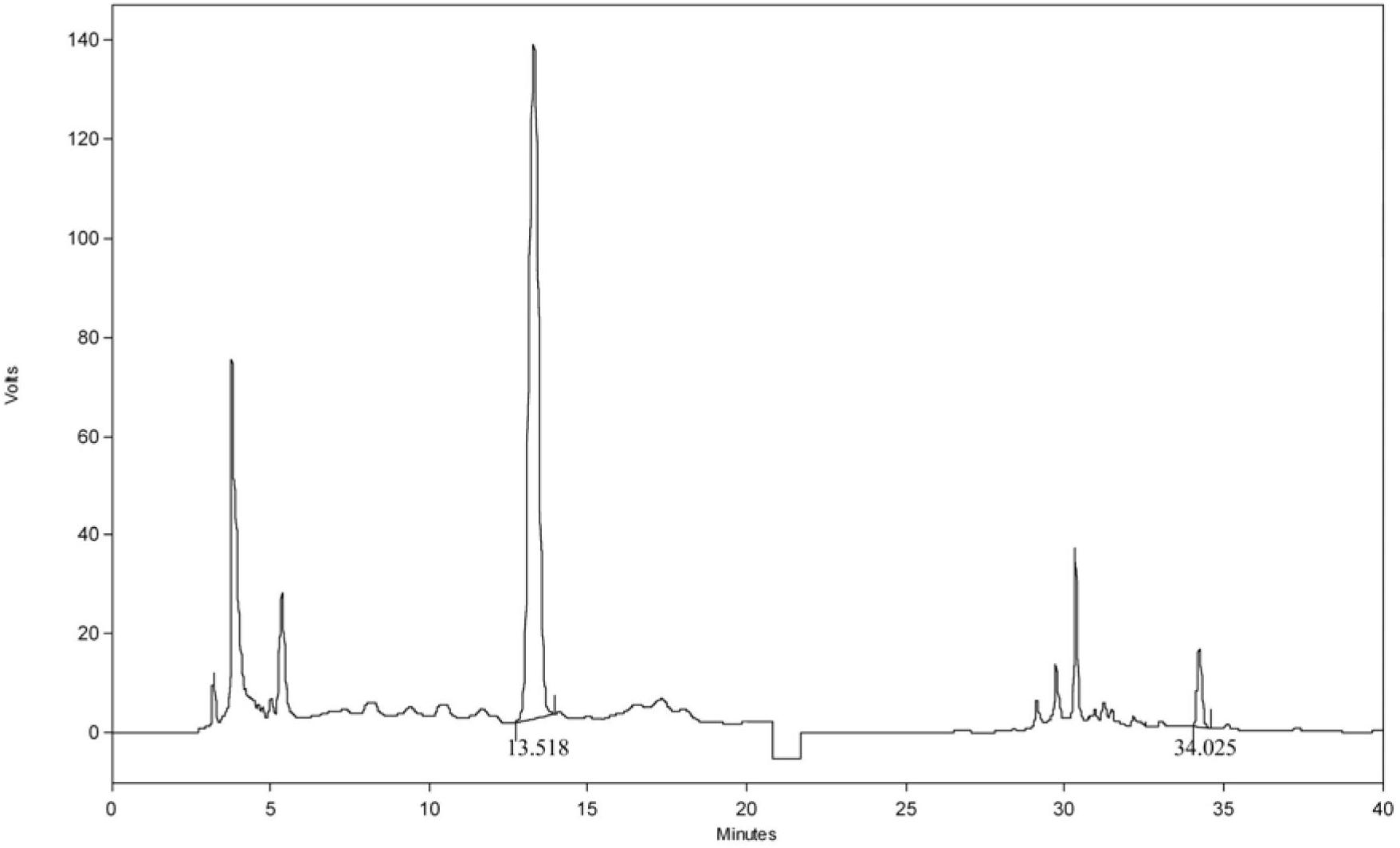
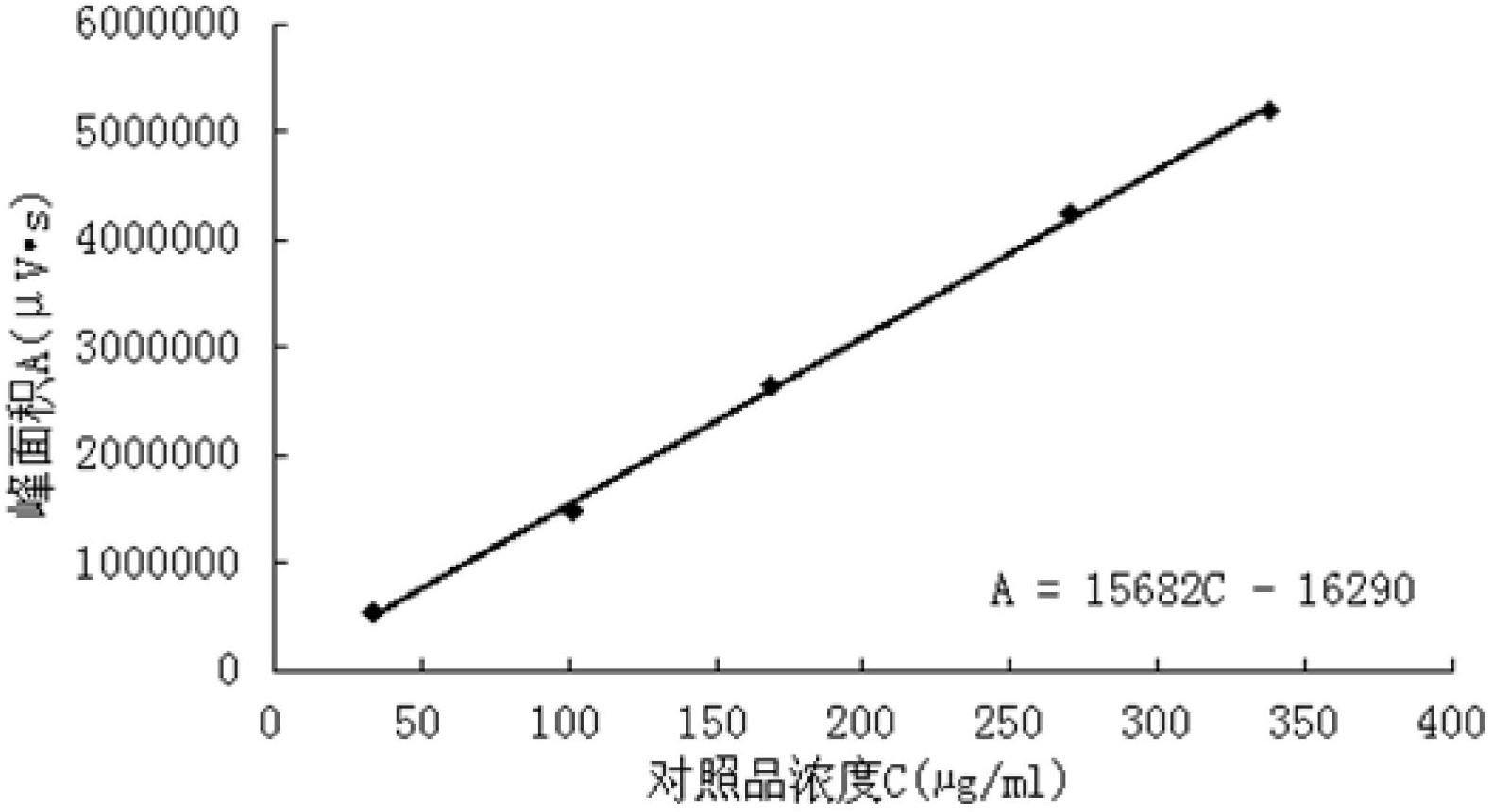
[0154] Embodiment 2: the assay method of Chinese medicine Qianlening Capsules
[0155] According to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition)
[0156] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; acetonitrile is used as mobile phase A, 1% glacial acetic acid aqueous solution is used as mobile phase B, and gradient elution method is adopted: 0min →20min→25min→50min→55min→60min, according to the above time period, acetonitrile and 1% glacial acetic acid aqueous solution are eluted at the same time, among which, acetonitrile: 1%→1%→24%→24%→100% →1%; volume ratio of 1% glacial acetic acid aqueous solution: 99%→99%→76%→76%→0%→99%, use the detection wavelength of 275nm in 0min→20min, change the detection wavelength to 403nm in 20min , 21min to balance the detector, 21min→60min to use a detection wavelength of 403nm. The number of theoretical plates should not be less ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com