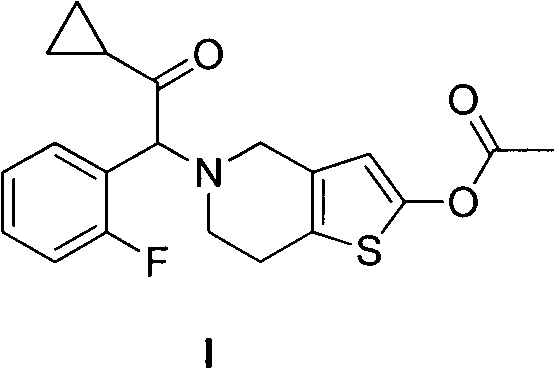
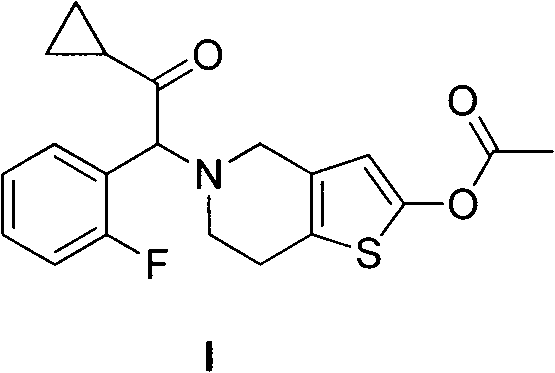
Method for preparing prasugrel
A technology of Grignard reagent and fluorobenzyl, which is applied in the field of antiplatelet drugs, can solve the problems of large amount of acetic anhydride and low coupling reaction yield, and achieve the goal of improving yield and product quality, short steps, and increasing yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
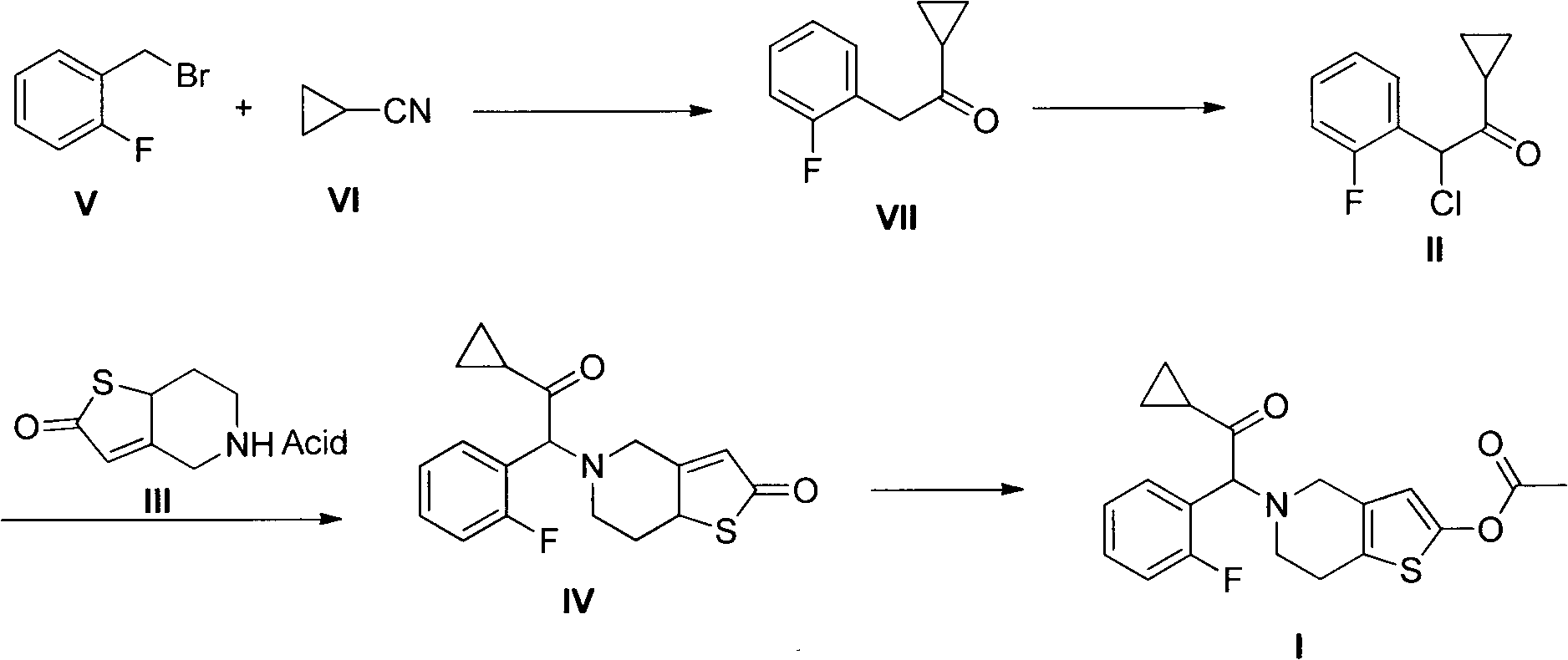
[0030] Add magnesium (9.6g, 0.4mol) and methyl tert-butyl ether (40mL) to a 1L three-necked flask, and at room temperature, dropwise add methyl Solution in tert-butyl ether (300 mL). After the dropwise addition, a solution of cyclopropanenitrile (26.8 g, 0.4 mol) in methyl tert-butyl ether (300 mL) was added dropwise to the reaction system. After the dropwise addition was completed, it was heated to reflux for 2 hours. Add saturated aqueous ammonium chloride solution (300mL) to the system, separate the organic layer, extract the aqueous layer with ethyl acetate (300mL), combine the organic layers, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain oily substance 3 (49.9g , 70%). 1 H NMR (CDCl 3 )δ: 0.88 ~ 0.93 (m, 2H, CH 2 ), 1.07~1.11 (m, 2H, CH 2 ), 2.00~2.04(m, 1H, CH), 3.90(s, 2H, CH 2 ), 7.07-7.29 (m, 4H, ArH).
Embodiment 2
[0032] Add 3 (131g, 0.74mol) and dichloromethane (500mL) into a 5L three-necked flask, add a solution of sulfonyl chloride (99.4g, 0.74mol) in dichloromethane (1000mL) dropwise at room temperature, and continue stirring for 1 hour after dropping . Add saturated aqueous sodium bicarbonate (1500mL), separate the organic layer, extract the aqueous layer with dichloromethane (1500mL), combine the organic layers, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to obtain oil 4 (125g, 82 %). 1 H NMR (CDCl 3 )δ: 0.94 ~ 1.20 (m, 4H, CH 2 CH 2 ), 2.09-2.13 (m, 1H, CH), 5.91 (s, 1H, CH), 7.12-7.48 (m, 4H, ArH).
Embodiment 3
[0034] 4 (106g, 0.5mol), 5,6,7,7α-tetrahydrothieno[3,2-c]pyridin-2(4H)-one p-toluenesulfonate (163g, 0.5 mol), potassium carbonate (138g, 1mol), sodium iodide (75g, 0.5mol), dimethyl sulfoxide (2500mL), heated at 60°C and stirred for 14 hours. Water was added and extracted with ethyl acetate (2 L). The organic layers were combined, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was crystallized from isopropyl ether (1 L) to obtain 5 (116 g, 70%) as a pale yellow solid. 1 H NMR (CDCl 3)δ: 0.70~1.08(m, 4H, CH 2 CH 2 ), 1.86~2.02(m, 1H, CH), 2.07~2.14(m, 1H, CH), 2.29~2.43, 2.52~2.58(m, 2H, CH 2 ), 2.85~2.88, 3.08~3.15 (m, 2H, CH 2 ), 3.93~4.00, 4.10~4.14 (m, 2H, CH 2 ), 4.87, 4.90 (s, s, 1H), 6.06, 6.08 (s, s, 1H), 7.15-7.38 (m, 4H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com