Amorphous cabazitaxel and preparation method thereof
A technology of cabazitaxel and amorphous substances, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of inconvenient industrial production, unstable physical and chemical state of products, and easy changes. Reach the effect of difficult crystal form conversion, suitable for industrial application, and low solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
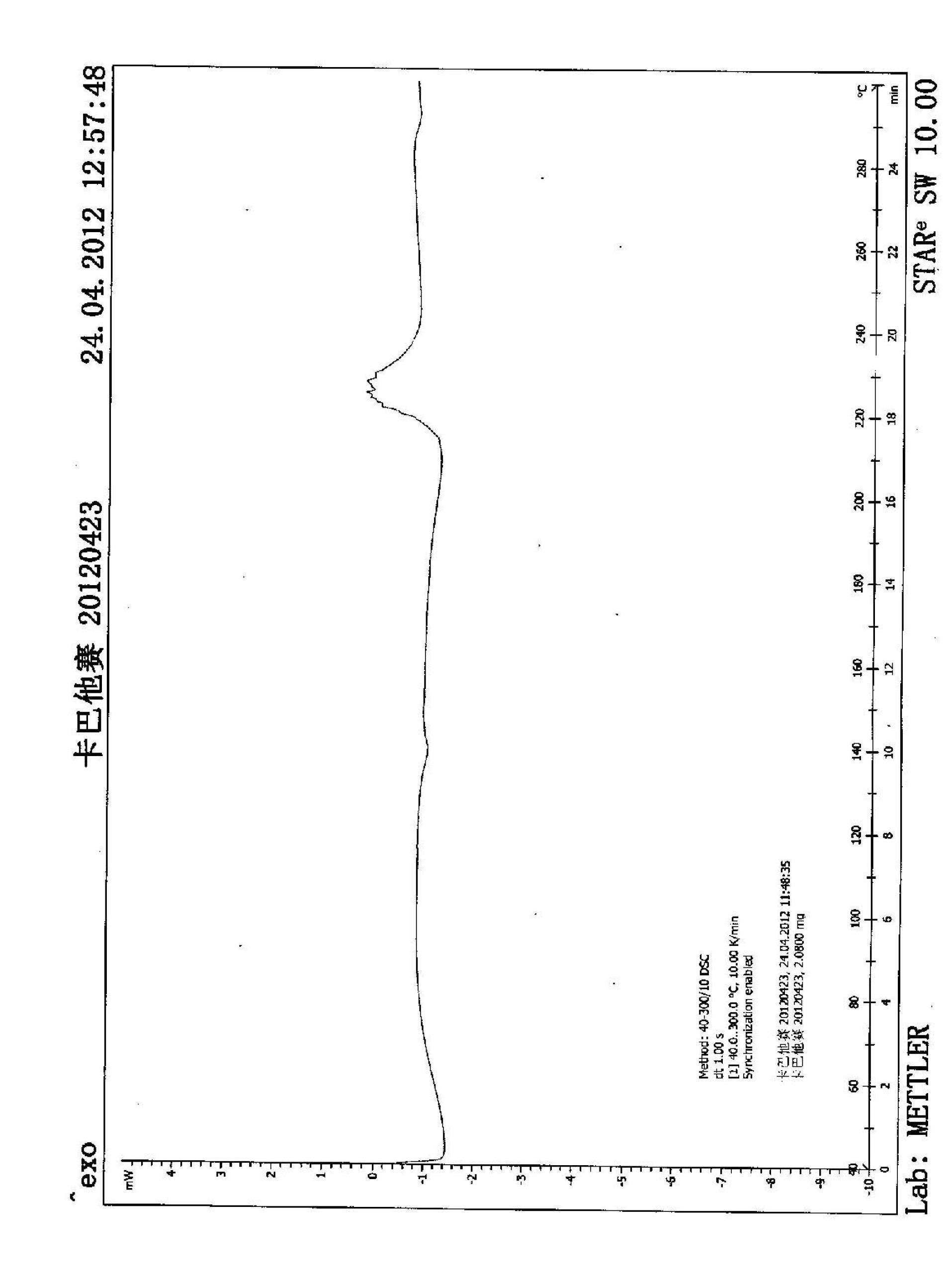
[0040] Weigh 500 mg of Cabazitaxel in Form A (prepared with reference to patent WO2005028462) and dissolve it in 2 ml of methanol. The above methanol solution of cabazitaxel was added dropwise to 18ml of water within 5 minutes at room temperature, and a large amount of white solids were precipitated immediately. After stirring at room temperature for 30 minutes, it was suction-filtered, washed with 5 ml of methanol / water=1 / 9 mixed solvent, and vacuum-dried to obtain 472 mg of a white solid with a yield of 94%. The X-ray diffraction spectrum of the sample is shown in figure 1 , see the DSC spectrum figure 2 , the compound was judged to be amorphous.
Embodiment 2
[0042] Weigh 500 mg of Cabazitaxel in Form B (synthesized with reference to patent CN101918385, the same below) and dissolve it in 2 ml of ethanol. The above ethanol solution of cabazitaxel was added dropwise to 18ml of water within 5 minutes at room temperature, and a large amount of white solids were precipitated immediately. After stirring at room temperature for 30 minutes, it was suction-filtered, washed with 5 ml of ethanol / water=1 / 9 mixed solvent, and dried in vacuo to obtain 481 mg of a white solid with a yield of 96%. The X-ray diffraction and DSC spectra of the sample are studied and compared, and it is determined that the product is amorphous.
Embodiment 3
[0044] Weigh 500 mg of Cabazitaxel in Form B and dissolve it in 2 ml of acetonitrile. The acetonitrile solution of the above-mentioned cabazitaxel was added dropwise to 18 ml of water within 5 minutes at room temperature, and a large amount of white solids were precipitated immediately. After stirring at room temperature for 30 minutes, it was filtered with suction, washed with 5 ml of acetonitrile / water=1 / 9 mixed solvent, and dried in vacuo to obtain 428 mg of a white solid with a yield of 86%. The X-ray diffraction and DSC spectra of the sample are studied and compared, and it is determined that the product is amorphous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com