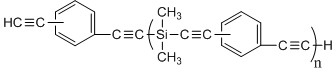
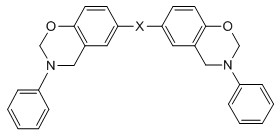
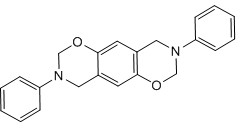
Bisphenol benzoxazine modified silicon-contained aryne resin and preparation method thereof
A technology of benzoxazine and silicon aryne, which is applied in the field of modified silicon-containing aryne resin and its preparation, can solve problems such as high brittleness, poor mechanical properties of composite materials, and unsatisfactory fiber adhesion, and achieve processing Good performance, low production cost, excellent physical and mechanical properties and high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Bisphenol A benzoxazine modified silicon-containing aryne resin (BA-a / PSA-30) and its preparation.
[0044] 1. Synthesis of bisphenol A benzoxazine (BA-a), whose structural formula is as follows Figure 7 shown.
[0045] Add 45.6 g (0.2 mol) of stoichiometric bisphenol A, 24.0 g (0.8 mol) of paraformaldehyde and 37.2 g (0.4 mol) of aniline into a 250 mL three-necked flask equipped with a stirring device, a nitrogen pouring tube and a condenser . Heat to 100°C under stirring, react for 20 min, after the reaction, dissolve the product in toluene (chloroform or ether), wash with 3 mol / L sodium hydroxide solution three times, then wash with deionized water until neutral, separate The organic layer was dried by adding anhydrous sodium sulfate, and finally the solvent was distilled off to obtain a light yellow solid with a yield of 70-75%. FTIR characteristic peak: 1496 cm -1 (1,2,4-trisubstituted benzene ring skeleton), 1228 cm -1 (C-O-C), 1162 cm -1 (C-N-C)...
Embodiment 2
[0051] Example 2: Hexafluorobisphenol A benzoxazine modified silicon-containing aryne resin (BAF-a / PSA-30) and its preparation.
[0052] 1. Synthesis of hexafluorobisphenol A benzoxazine (BAF-a), whose structural formula is as follows Figure 8 shown.
[0053] Into a 250 mL three-neck flask equipped with a stirring device, a nitrogen pouring tube and a condenser tube, 67.2 g (0.2 mol) of hexafluorobisphenol A, 24.0 g (0.8 mol) of paraformaldehyde and 37.2 g (0.4 mol). Heat to 100°C under stirring, react for 20 min, after the reaction, dissolve the product in toluene (chloroform or ether), wash with 3 mol / L sodium hydroxide solution three times, then wash with deionized water until neutral, separate The organic layer was dried by adding anhydrous sodium sulfate, and finally the solvent was distilled off to obtain a yellow solid with a yield of 70-75%. FTIR characteristic peak: 1500 cm -1 (1,2,4-trisubstituted benzene ring skeleton), 1244 cm -1 (C-O-C), 1129 cm -1 (C-N-C),...
Embodiment 3
[0057] Example 3: Diphenyl ether type benzoxazine modified silicon-containing aryne resin (BO-a / PSA-30) and its preparation.
[0058] 1. Synthesis of diphenyl ether-type benzoxazine (BO-a), whose structural formula is as follows Figure 9 shown.
[0059] Into a 250 mL three-neck flask equipped with a stirring device, a nitrogen pouring tube and a condenser, add stoichiometric 40.4 g (0.2 mol) of 4,4'-dihydroxydiphenyl ether, 24.0 g (0.8 mol) of paraformaldehyde and Aniline 37.2 g (0.4 mol). Heat to 100°C under stirring, react for 20 min, after the reaction, dissolve the product in toluene (chloroform or ether), wash with 3 mol / L sodium hydroxide solution three times, then wash with deionized water until neutral, separate The organic layer was dried by adding anhydrous sodium sulfate, and finally the solvent was distilled off to obtain a reddish-brown solid with a yield of 70-75%. FTIR characteristic peak: 1489 cm -1 (1,2,4-trisubstituted benzene ring skeleton), 1220 cm -1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| gel time | aaaaa | aaaaa |
| gel time | aaaaa | aaaaa |
| gel time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com