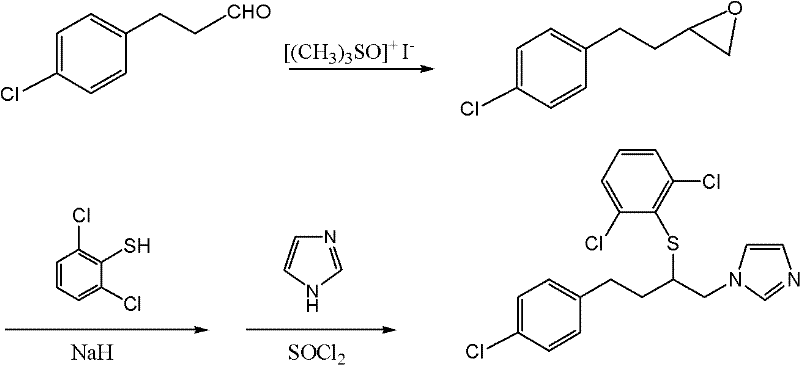
Preparation method for important intermediates of butoconazole nitrate
A volume ratio, chlorobenzyl chloride technology, applied in the direction of magnesium organic compounds, organic chemistry, etc., can solve the problems that are not suitable for industrialization, and achieve the effects of easy control of the reaction, reduction of side reactions, and safe operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
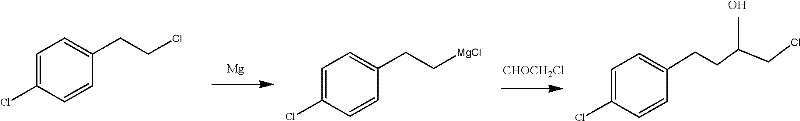
[0025] The preparation method of embodiment 1 4-chlorobenzylmagnesium chloride (formula I compound)
[0026] Add 5g of magnesium powder, 30ml of ether, and 1 grain of iodine into a 500ml three-necked bottle, and heat up to 30°C. Add 33g of p-chlorobenzyl chloride, 20ml of diethyl ether, and 200ml of methyl tert-butyl ether into the dropping funnel, drop about 10ml into the reaction bottle to initiate the reaction, then control the temperature of 30-40°C to slowly add all the materials dropwise, and then Warm reaction for 2 hours to obtain a mixed solution of bright green Grignard reagent 4-chlorobenzylmagnesium chloride (compound of formula I), diethyl ether and methyl tert-butyl ether. Measure the concentration of 4-chlorobenzylmagnesium chloride to be 0.843mol / l, volume 247ml, yield: 93.8% (concentration determination method: take about 0.5g of sample (sealed and weighed), place in a conical flask, add water 10-15ml, add Quantitatively dilute hydrochloric acid, add indicato...
Embodiment 2
[0027] The preparation method of embodiment 2 4-chlorobenzylmagnesium chloride (formula I compound)
[0028] Add 5g of magnesium powder, 40ml of ether, and 1 grain of iodine into a 500ml three-necked flask, and heat up to 30°C. Add 33g of p-chlorobenzyl chloride, 20ml of diethyl ether, and 180ml of methyl tert-butyl ether into the dropping funnel, drop about 10ml into the reaction bottle to initiate the reaction, then control the temperature of 30-40°C to slowly add all the materials dropwise, and then Warm reaction for 2 hours to obtain a mixed solution of bright green Grignard reagent 4-chlorobenzylmagnesium chloride (compound of formula I), diethyl ether and methyl tert-butyl ether. Measure 4-chlorobenzylmagnesium chloride concentration and be 0.827mol / l, volume 235ml, yield: 93.3% (concentration determination method is the same as above)
Embodiment 3
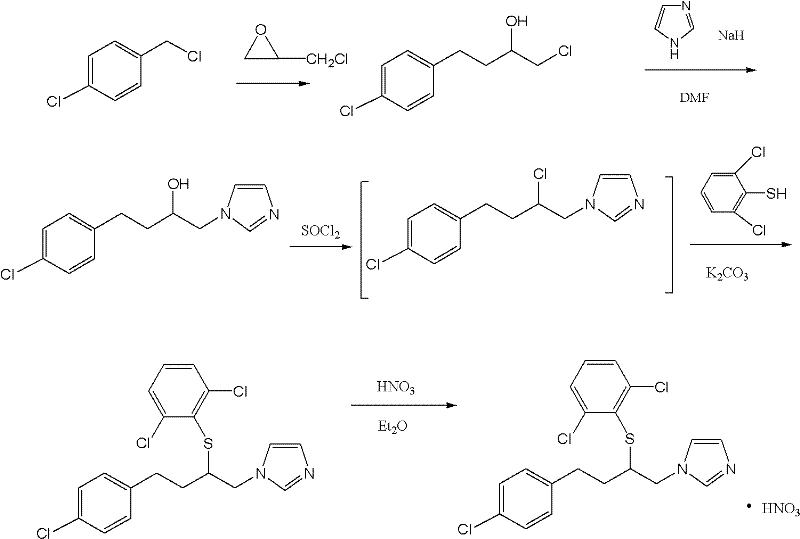
[0029] Example 3 The preparation method of 1-[2-chloro-4-(4-chlorophenyl)-n-butyl]imidazole (compound of formula III)
[0030] Add 1-[4-(4-chlorophenyl)-2-hydroxy-n-butyl]imidazole (compound 2) 18g (0.072mol), dichloromethane 100ml, thionyl chloride 9.42g into a 250ml three-necked flask (0.079mol), heated and refluxed for 1 hour, lowered to room temperature, added 10ml of water, adjusted pH=8 with saturated aqueous sodium carbonate solution, separated the water layer, concentrated the organic layer to dryness, and obtained brown oil 1-[2-chloro-4 -(4-Chlorophenyl)-n-butyl]imidazole (compound of formula III) 17.3 g (purity measured by HPLC: 98.6%), yield 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com