Preparation method of 5-(piperazine-1-group) benzofuran-2-carboxylic acid ethyl ester
A technology of benzofuran and ethyl carboxylate, which is applied in the field of preparation of ethyl 5-benzofuran-2-carboxylate, can solve the problems of low yield, no post-treatment method and yield given, and achieve The effect of improving yield and product purity, simple and reliable method, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
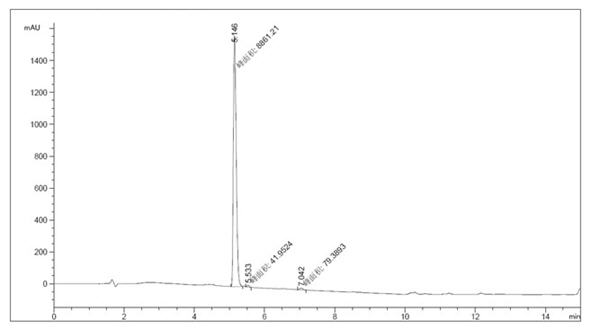
[0025] 1 Add 50g of ethyl 5-aminobenzofuran-2-carboxylate, 50g of N,N-bis(2-chloroethyl)amine hydrochloride, 60g of anhydrous sodium carbonate, and tetrabutylammonium bromide into the reaction flask 4g, 500ml of n-butanol, heated and refluxed for 5 hours, filtered with suction, washed the filter cake with water to remove inorganic salts, and dried under reduced pressure at 40-50°C to obtain 60g of off-white solid with a yield of 89% and a purity of 98.6%. HPLC profile such as figure 1 shown. See Table 1 for specific data.
[0026] Table 1 Specific data of each spectrum
[0027] peak Retention time (min) Types of Peak width (min) Peak area (mAU*s) Peak height (mAU) Peak area% 1 5.146 MM 0.0938 8861.20703 1573.71216 98.6491 2 5.533 MM 0.0645 41.95242 10.83328 0.4670 3 7.042 MM 0.1209 79.38930 10.94166 0.8838 total 8982.54875 1595.48709
Embodiment 2
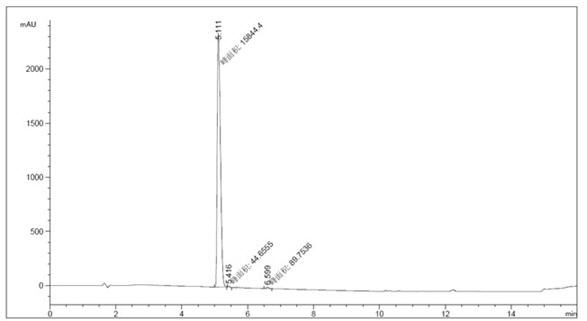
[0029] 1 Add 50g of ethyl 5-aminobenzofuran-2-carboxylate, 50g of N,N-bis(2-chloroethyl)amine hydrochloride, 57g of triethylamine, and 4g of tetrabutylammonium bromide into the reaction flask , toluene 500ml, heat up and react for 2 hours, filter with suction, wash the filter cake with water to remove inorganic salts, dry under reduced pressure at 40-50°C to obtain 56g of off-white solid, yield 84%, purity 99.1%. HPLC profile such as figure 2 shown. See Table 2 for specific data.
[0030] Table 2 Specific data of each spectrum
[0031] peak Retention time (min) Types of Peak width (min) Peak area (mAU *s) Peak height (mAU) Peak area% 1 5.111 MM 0.1124 1.58444e4 2349.64648 99.1588 2 5.416 MM 0.0589 44.65551 12.63103 0.2795 3 6.599 MM 0.1049 89.75358 14.26618 0.5617 total 1.59788e4 2376.54369
Embodiment 3
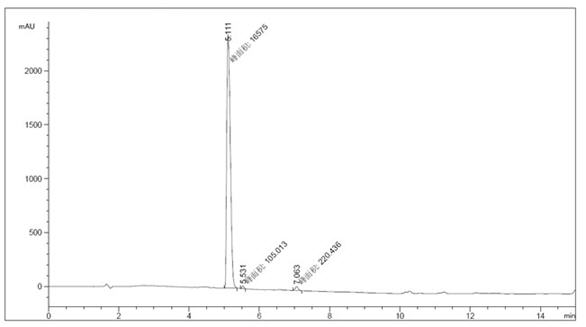
[0033] 1 Add 50g of ethyl 5-aminobenzofuran-2-carboxylate, 50g of N,N-bis(2-chloroethyl)amine hydrochloride, 4g of tetrabutylammonium chloride, and 500ml of distilled water into the reaction flask, and divide Add 60 g of anhydrous sodium carbonate in 5 batches, with an interval of 30 minutes between each batch. After the addition, raise the temperature and reflux for 3 hours, filter with suction, and dry the filter cake under reduced pressure at 40-50°C to obtain 54 g of off-white solid, with a yield of 81% and a purity of 98%. HPLC profile such as image 3 . See Table 3 for specific data.
[0034] Table 3 Specific data of each spectrum
[0035] peak Retention time (min) Types of Peak width (min) Peak area (mAU *s) Peak height (mAU) Peak area% 1 5.111 MM 0.1176 1.65750e4 2349.26392 98.0743 2 5.531 MM 0.0692 105.01313 25.28586 0.6214 3 7.063 MM 0.0989 220.43629 37.15370 1.3043 total 1.69005e4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com