Ferrate/polyaniline magnetic nanometer catalytic agent and preparation method thereof
A magnetic nano and ferrite technology, applied in the field of material chemistry, can solve the problems of non-magnetism, non-selective adsorption performance of catalysts, and inability to easily recycle and recycle, and achieve excellent adsorption performance, excellent visible light catalytic activity, excellent Effect of Selective Adsorption Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
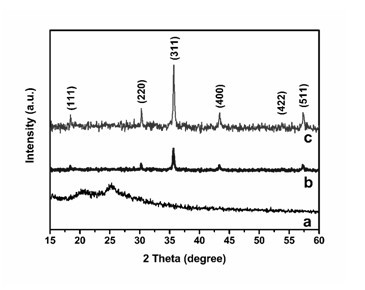
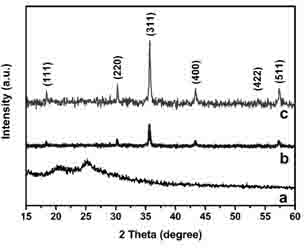
Image
Examples
Embodiment 1
[0026] Example 1: Cobalt ferrite / polyaniline magnetic nano-catalyst, the preparation method that polyaniline content is 5wt%, comprises the following steps:
[0027] In the first step, ferric nitrate and cobalt nitrate with a mol ratio of 2:1 were stirred in ethanol for 90min;
[0028] In the second step, sodium hydroxide with a mol ratio of 80:1 to the cobalt nitrate added in step one is added to the solution obtained in the first step, and stirred for 60 min;
[0029] In the third step, react the mixed solution in the second step at 120°C for 12 hours, centrifuge and dry to obtain nano-cobalt ferrite;
[0030] The 4th step, the nano cobalt ferrite that ammonium persulfate and the 3rd step gained are dispersed in the hydrochloric acid solution that concentration is 0.5mol / L;
[0031] In the fifth step, add aniline with a molar ratio of 0.1 to the ammonium persulfate added in the fourth step to the system obtained in the fifth step, react at a temperature of 0°C for 24 h, a...
Embodiment 2
[0033] Example 2: Nickel ferrite / polyaniline magnetic nano-catalyst, the preparation method that polyaniline content is 90wt%, comprises the following steps:
[0034] The first step, be that ferric nitrate and nickel chloride of 2:1 are stirred 10min in ethanol with mol ratio;
[0035] In the second step, ammonia water having a molar ratio of 60:1 to the divalent transition metal salt added in step 1 is added to the solution obtained in the first step, and stirred for 10 min;
[0036] In the third step, the mixed solution in the second step was reacted at 140° C. for 24 h, then centrifuged and dried to obtain nano-nickel ferrite;
[0037] The 4th step, ammonium persulfate and the nano-nickel ferrite of the 3rd step gained are dispersed in the sulfuric acid solution that concentration is 1mol / L;
[0038] In the fifth step, aniline with a molar ratio of 10 to the ammonium persulfate added in the fourth step is added to the system obtained in the fifth step, and the reaction i...
Embodiment 3
[0039] Example 3: Copper ferrite / polyaniline magnetic nano-catalyst, the preparation method that polyaniline content is 10wt%, comprises the following steps:
[0040] In the first step, ferric nitrate and copper sulfate with a molar ratio of 2:1 were stirred in ethanol for 30 min;
[0041] In the second step, urea with a mol ratio of 40:1 to the divalent transition metal salt added in step one is added to the solution obtained in the first step, and stirred for 25 min;
[0042] In the third step, react the mixed solution in the second step at 160°C for 20 hours, then centrifuge and dry to obtain nano-copper ferrite;
[0043] The 4th step, the nano-copper ferrite that ammonium persulfate and the 3rd step gained are dispersed in the phosphoric acid solution that concentration is 2mol / L;
[0044] In the fifth step, aniline with a molar ratio of 1 to the ammonium persulfate added in the fourth step is added to the system obtained in the fifth step, and the reaction is carried out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com