Cobalt-doped carbon-coated ferric fluoride anode material and preparation method thereof
A cathode material, iron fluoride technology, applied in battery electrodes, electrical components, circuits, etc., can solve problems such as poor electrochemical cycle performance, and achieve the effects of low cost, excellent electrochemical cycle performance, and easy industrial promotion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh Fe according to the molar ratio of n(Fe):n(Co)=0.95:0.05 2 o 3 and Co 2 o 3 About 10g, after mixing evenly, add 40wt% HF at a molar ratio of 1:4 under normal temperature and constant stirring, put it into a sealed polytetrafluoroethylene container, and stir at 25°C for 4h. Then the temperature was raised to 75° C. to fully react until a pink solid was formed. The obtained material was washed with ethanol three times and dried at 60° C. in an air atmosphere for 10 h, and then the material was vacuum-dried at 160° C. for 5 h. Obtain Fe with a purity greater than 96% 0.95 co 0.05 f 3 (H 2 O) 0.33 product, mix it with 15wt% acetylene black, ball mill for 3h, keep vacuum at 150°C for 6h, and pass through a 400-mesh sieve to obtain Fe 0.95 co 0.05 f 3 (H 2 O) 0.33 / C Composite.
Embodiment 2
[0035] Weigh Fe according to the molar ratio of n(Fe):n(Co)=0.97:0.03 2 o 3 and Co 3 o 4 About 10g, after mixing evenly, add 30wt%% HF at a molar ratio of 1:6 under normal temperature and constant stirring, put it into a sealed polytetrafluoroethylene container, and stir at 30°C for 3h. Then the temperature was raised to 80°C to fully react until a gray-black solid was formed. The obtained material was washed with ethanol three times and dried at 80°C in an air atmosphere for 6 hours, and then the material was vacuum-dried at 170°C for 4 hours. Obtain Fe with a purity greater than 96% 0.97 co 0.03 f 3 (H 2 O) 0.33 product, mix it with 20wt% acetylene black, ball mill for 3h, keep vacuum at 160°C for 5h, and pass through a 400-mesh sieve to obtain Fe 0.97 co 0.03 f 3 (H 2 O) 0.33 / C Composite.
Embodiment 3
[0037] Weigh industrial Fe(OH) according to the molar ratio of n(Fe):n(Co)=0.92:0.08 3 and CoCO 3 About 10 g was added with 20 wt% HF at a molar ratio of 1:8, put into a sealed polytetrafluoroethylene container, and stirred at 25°C for 6 hours to make it evenly mixed. Then the temperature was raised to 75° C. to fully react until a pink solid was formed. The obtained material was washed with ethanol three times and dried at 80° C. for 6 h in an air atmosphere, and then vacuum-dried at 180° C. for 2 h. The product is taken out, pulverized, and passed through a 400-mesh sieve to obtain Fe with a purity greater than 96%. 0.92 co 0.08 f 3 (H 2 O) 0.33 product. It was ball milled with 15wt% acetylene black for 3 hours, kept in vacuum at 180°C for 2 hours, and passed through a 400-mesh sieve to obtain Fe 0.92 co 0.08 f 3 (H 2 O) 0.33 / C Composite.
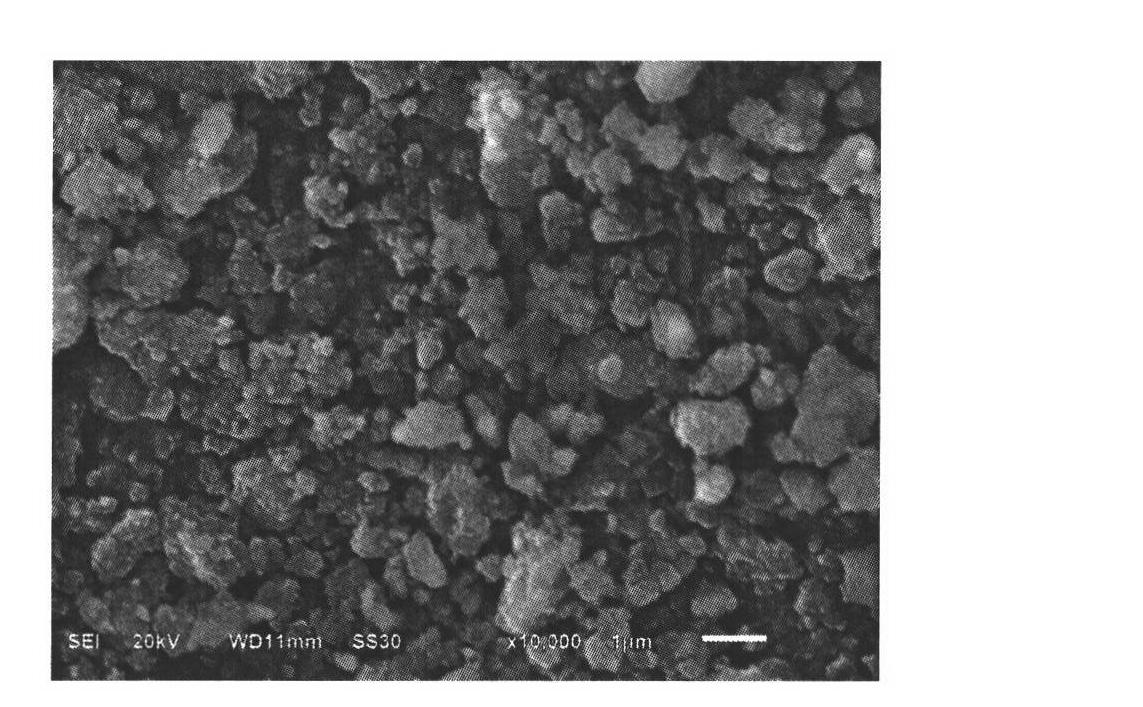
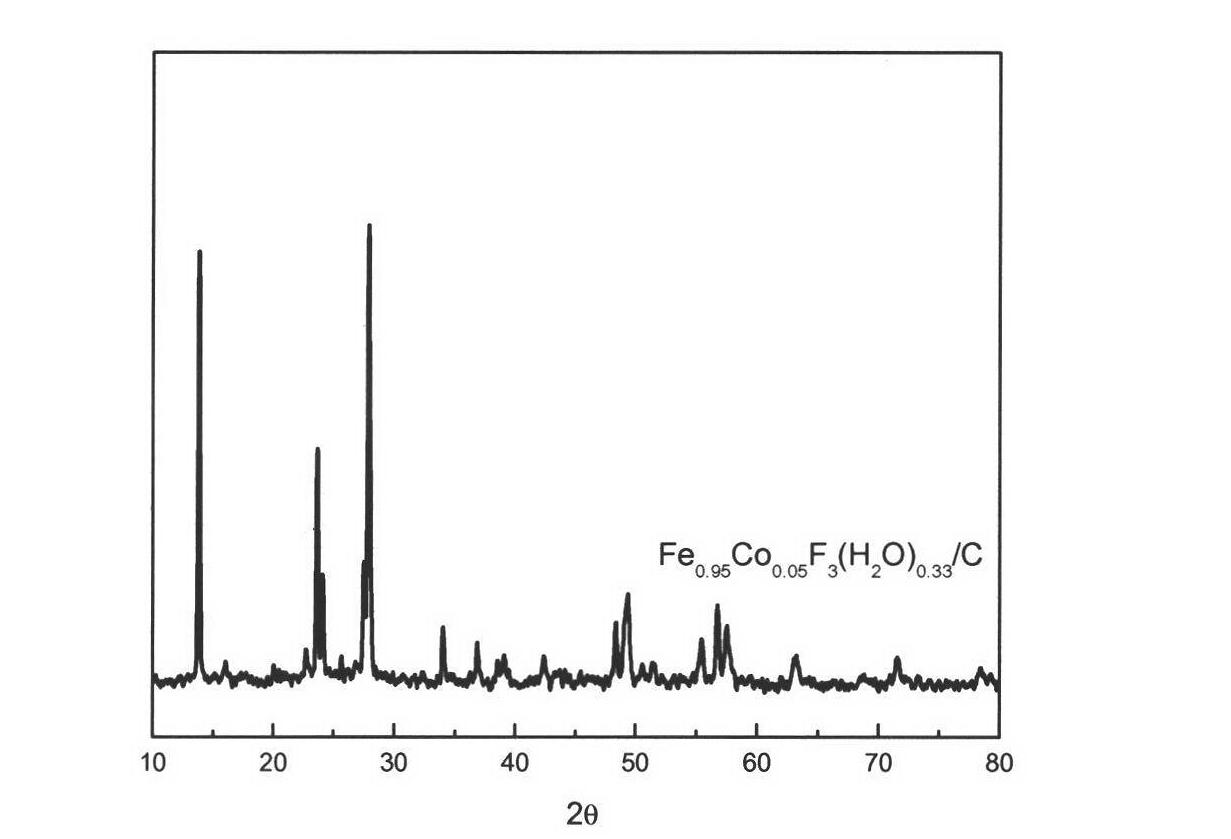
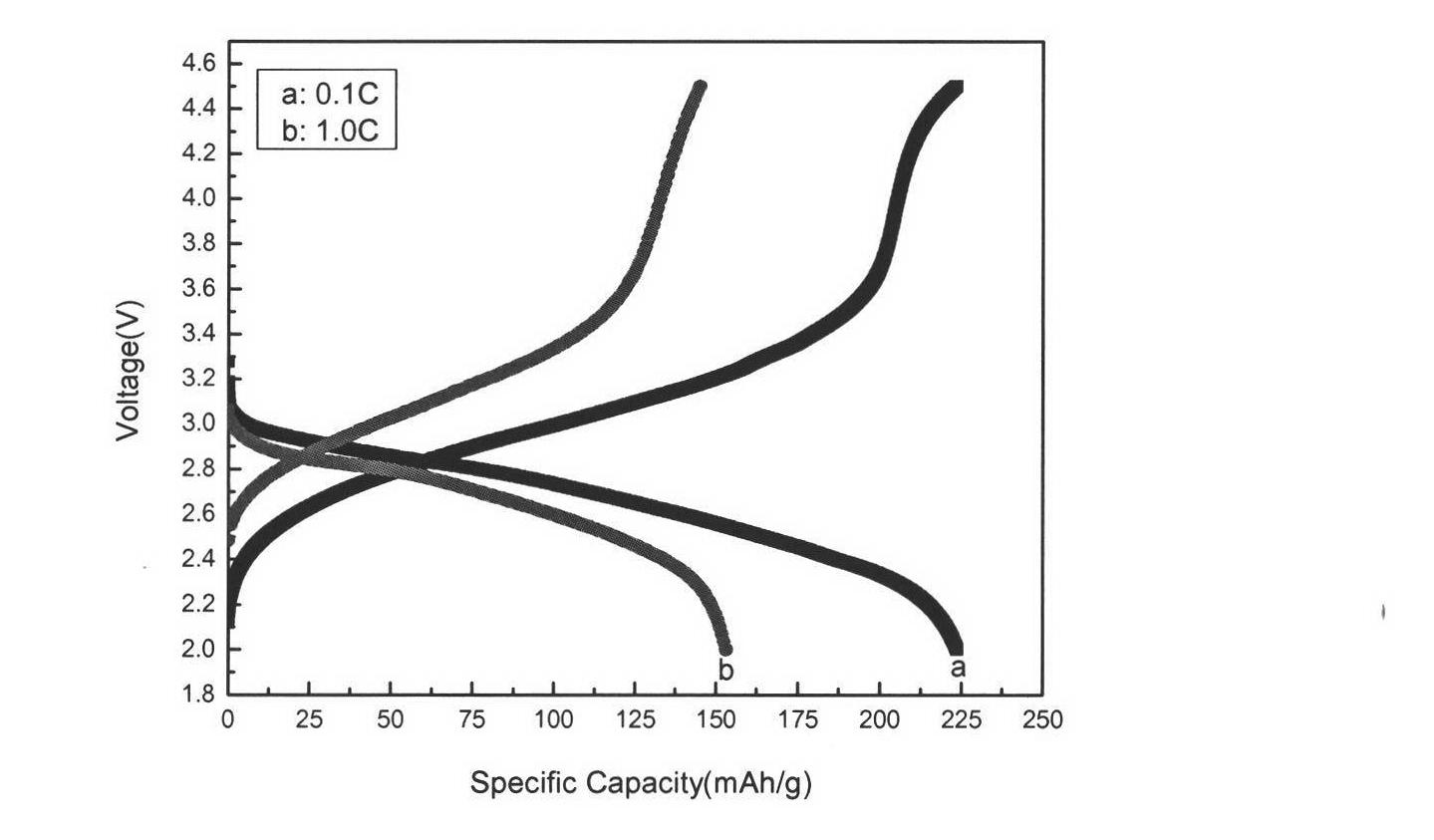
[0038] Such as figure 1 As shown, it can be seen from the figure that the prepared Fe 0.95 co 0.05 f 3 (H 2 O) 0.33 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com