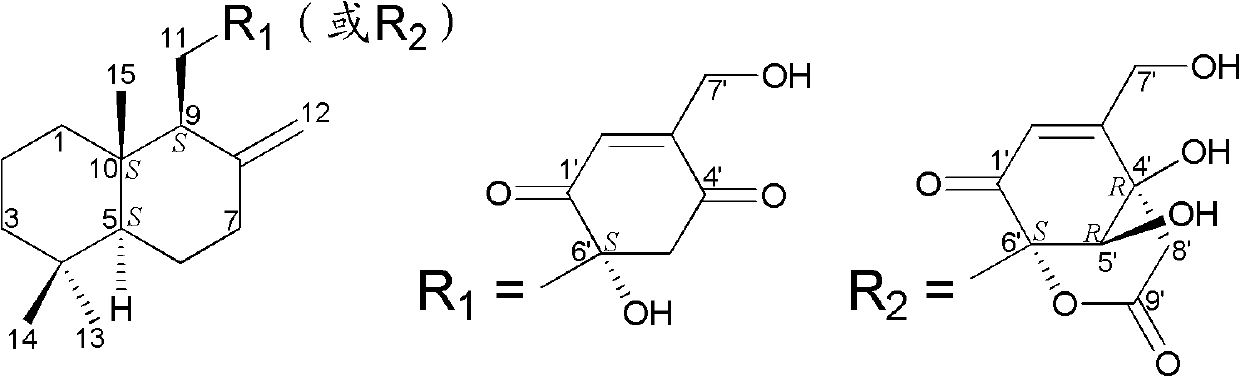
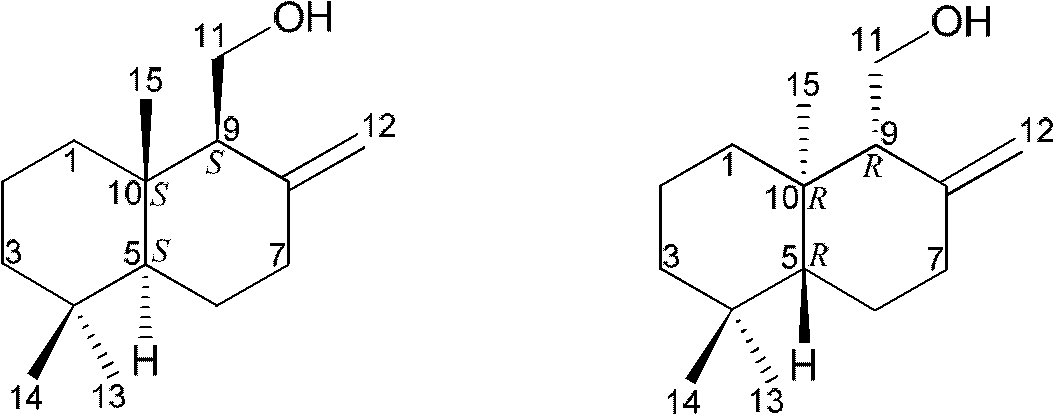
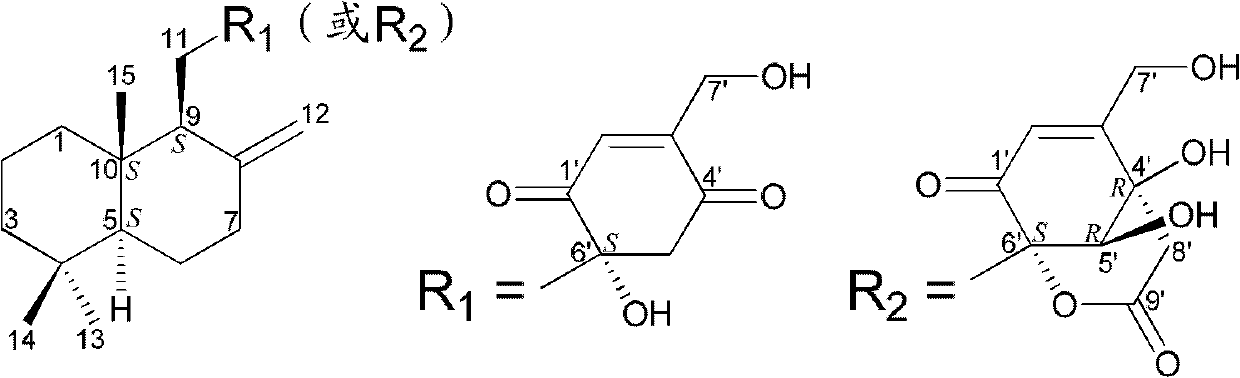
Drimane-type sesquialter terpene cyclohexenone derivative, preparation method thereof and application
A technology of microorganisms and microbial strains, applied in the field of medicine and chemical industry, to achieve the effect of inhibiting proliferation and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Microbial fermentation culture and the preparation of compound 1 and compound 2
[0061] 1. Fermentation culture and extraction of fermented products
[0062] 1.1) Production strain
[0063] The toxin-producing bacteria used for the fermentation and production of compounds 1 and 2 in this example is the Penicillium purpurogenum BD-1-6 strain preserved in the General Microorganism Center of the China Microbiological Culture Collection Management Committee, and the preservation number is 5525. CGMCC No.5525.
[0064] 1.2) Fermentation culture
[0065]From the PDA medium (composition: 2% glucose, 2% agar, 1.5% NaCl, prepared with 20% potato boiling liquid) test tube slant , Scraped an appropriate amount of spores under aseptic conditions with an inoculation loop, streaked and inoculated them on a newly prepared PDA solid medium plate, and activated them in a 28°C incubator for 4 days. From the slant of the test tube that has been activated and cultivated...
Embodiment 2
[0082] Embodiment 2: Antitumor activity test of compound 1 and compound 2
[0083] 1. Experimental materials
[0084] 1) Preparation of the tested sample solution
[0085] The tested samples were pure compound 1 and compound 2 isolated and refined in the above-mentioned Example 1. 5-Fluorouracil (Aladdin Reagent Co., Ltd., batch number 5402) and docetaxel trihydrate (Beijing Qimiwo Technology Co., Ltd., batch number 20110326) were used as positive control samples. Precisely weigh an appropriate amount of samples, and use DMSO to prepare solutions of required concentrations for testing activity.
[0086] 2) Subculture of cell lines and cells
[0087] The activity test adopts human chronic myeloid leukemia K562 cell line, human acute promyelocytic leukemia HL-60 cell line, human cervical cancer HeLa cell line, human gastric cancer BGC-823 line, human breast cancer MCF-7 line (the above cells Both can be purchased commercially, for example, from Shanghai Yaji Biotechnology ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com