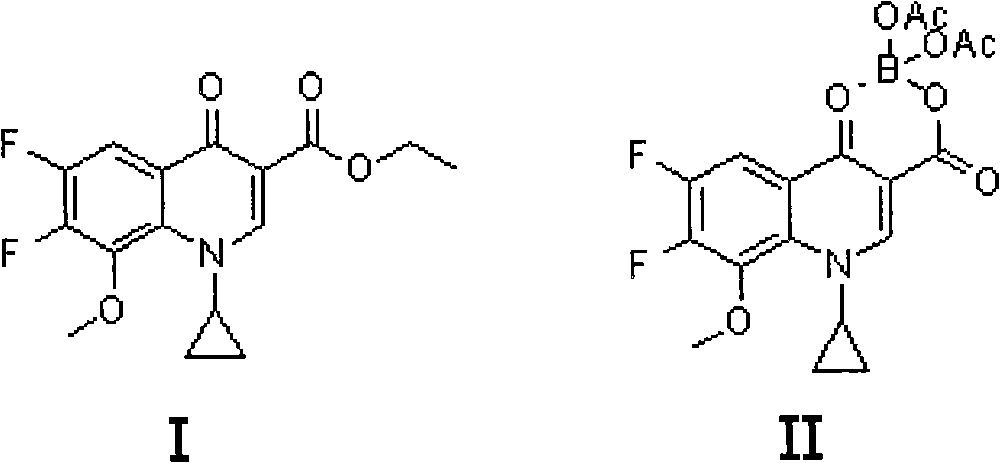
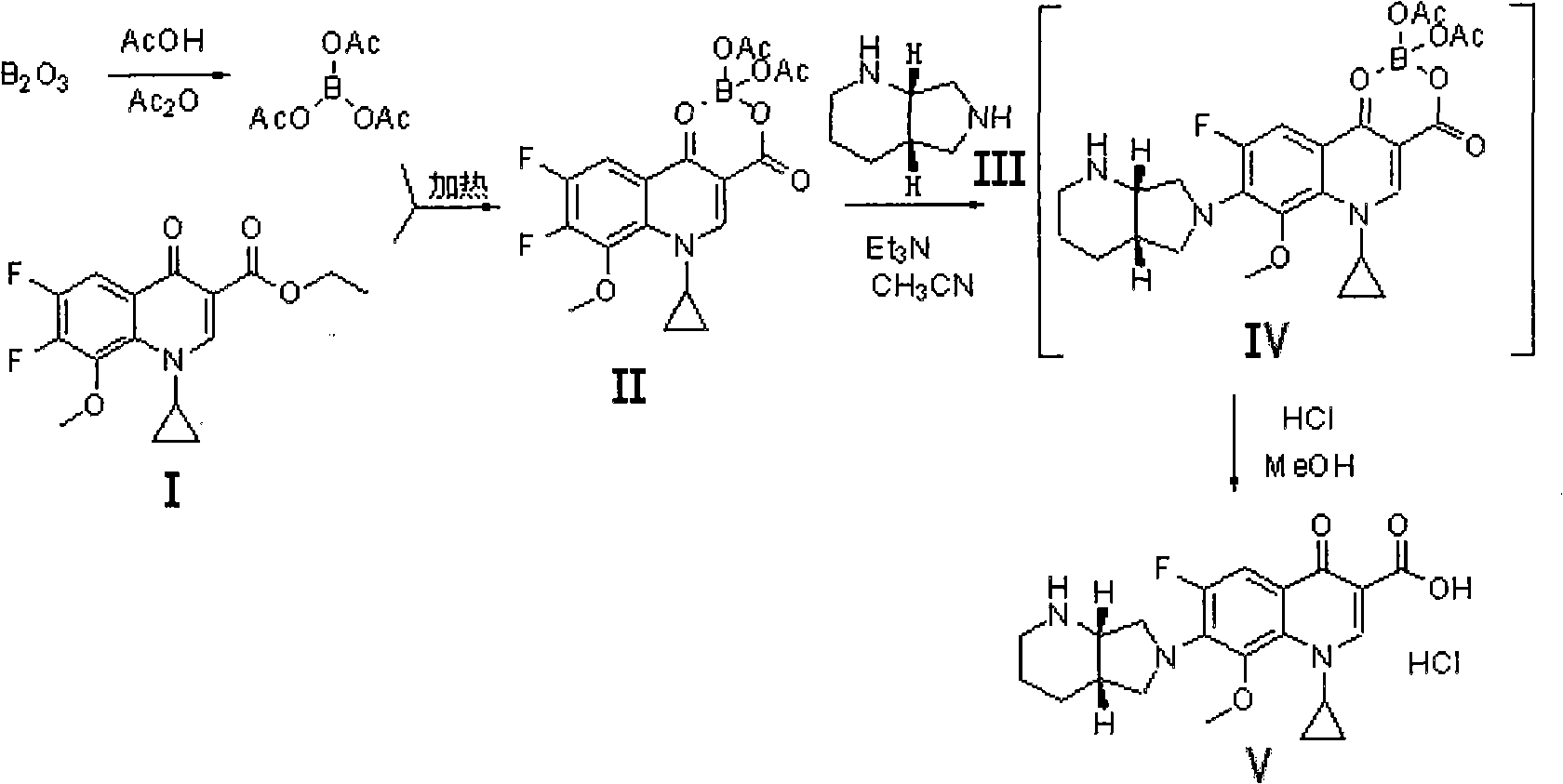
Method for preparing moxifloxacin or its medicinal salt and its intermediate
A technology of medicinal salt and cyclopropyl, which is applied in the field of compound preparation, can solve the problems of product moxifloxacin color influence, intermediate product color deepening, equipment and human injury, etc., and achieve low requirements for reaction equipment and good reproducibility , easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 , O 4 -Preparation of boron diacetate (compound II)
[0032] Dissolve powdery boron trioxide (63.9g, or 0.918mol) in acetic anhydride (511.2mL) and acetic acid (319.5mL), then react at 90-100°C for 2h, then cool, and add quinolinecarboxylic acid Cyclic ester (Compound I) (517g, namely 1.6mol), reacted at 50-60°C for 5h, then cooled to 40°C, added anisole (1.1L), cooled to 15-20°C while stirring, and then Stirring at this temperature for 2 hours, a large amount of solid precipitated out, filtered, washed with anisole, and dried to obtain the compound II product (576 g, yield 91.1%, HPLC purity 99.5%).
Embodiment 2
[0033] Example 2: 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 , O 4 -Preparation of boron diacetate
[0034] Dissolve powdery boron trioxide (63.9g, ie 0.918mol) in acetic anhydride (958.5mL) and acetic acid (511.2mL), then react at 95-105°C for 4h, then cool to 60-70°C, add formula (I) Compound quinolinecarboxylic acid cyclic ester (790g, namely 2mol), reacted at 60-70°C for 8h, then cooled to 40-50°C, added isopropyl ether (1.2L), and cooled to 15-20°C, and then stirred at this temperature for 2 hours, a large amount of solid precipitated, filtered, washed with isopropyl ether, and dried in vacuo to obtain Compound II (668g, yield 92.0%, HPLC purity 99.8%).
Embodiment 3
[0035] Example 3: 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 , O 4 -Preparation of boron diacetate
[0036] Dissolve powdery boron trioxide (63.9g, namely 0.918mol) in acetic anhydride (639mL) and acetic acid (400mL), then react at 110-120°C for 3h, then cool to 70-80°C, add formula (I ) Compound quinoline carboxylic acid cyclic ester (711g, namely 1.8mol), reacted at 70-80°C for 6h, then cooled to 40-50°C, added methyl tert-butyl ether (1L), cooled while stirring to 15-20°C, then stirred at this temperature for 2h, a large amount of solid precipitated out, filtered, washed with methyl tert-butyl ether, and dried in vacuo to obtain compound II product (652g, yield 91.6%, HPLC purity 99.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com