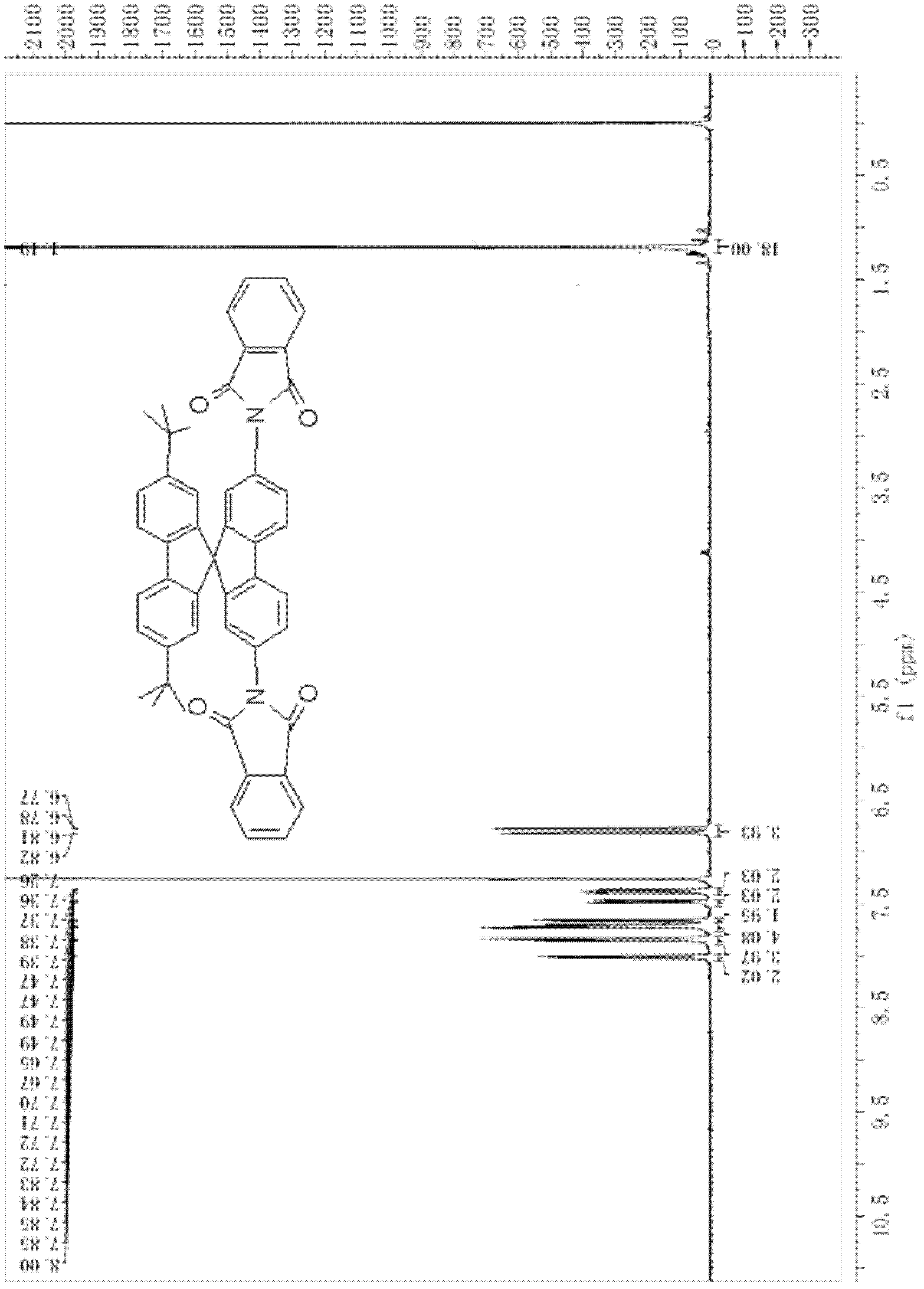
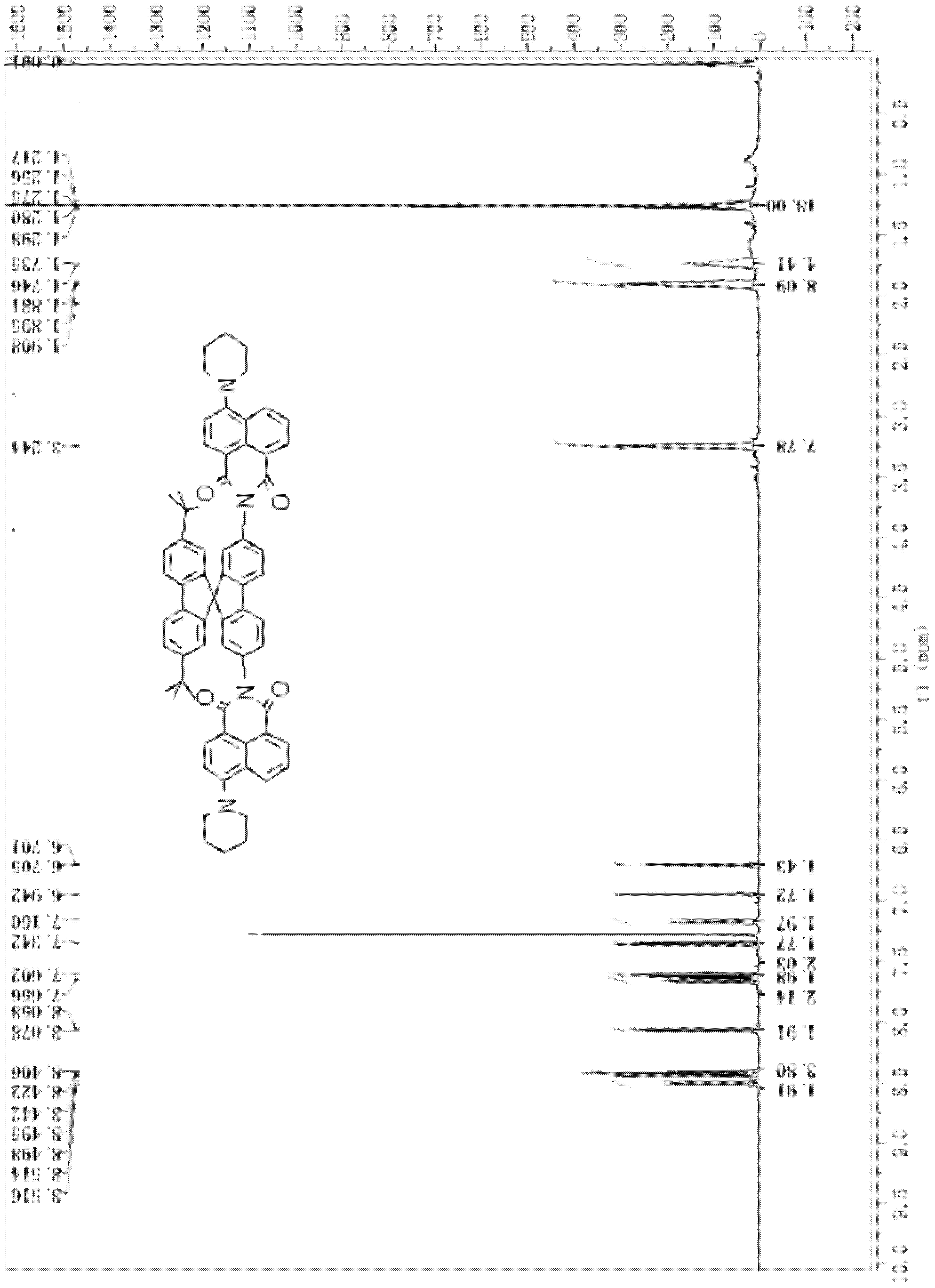
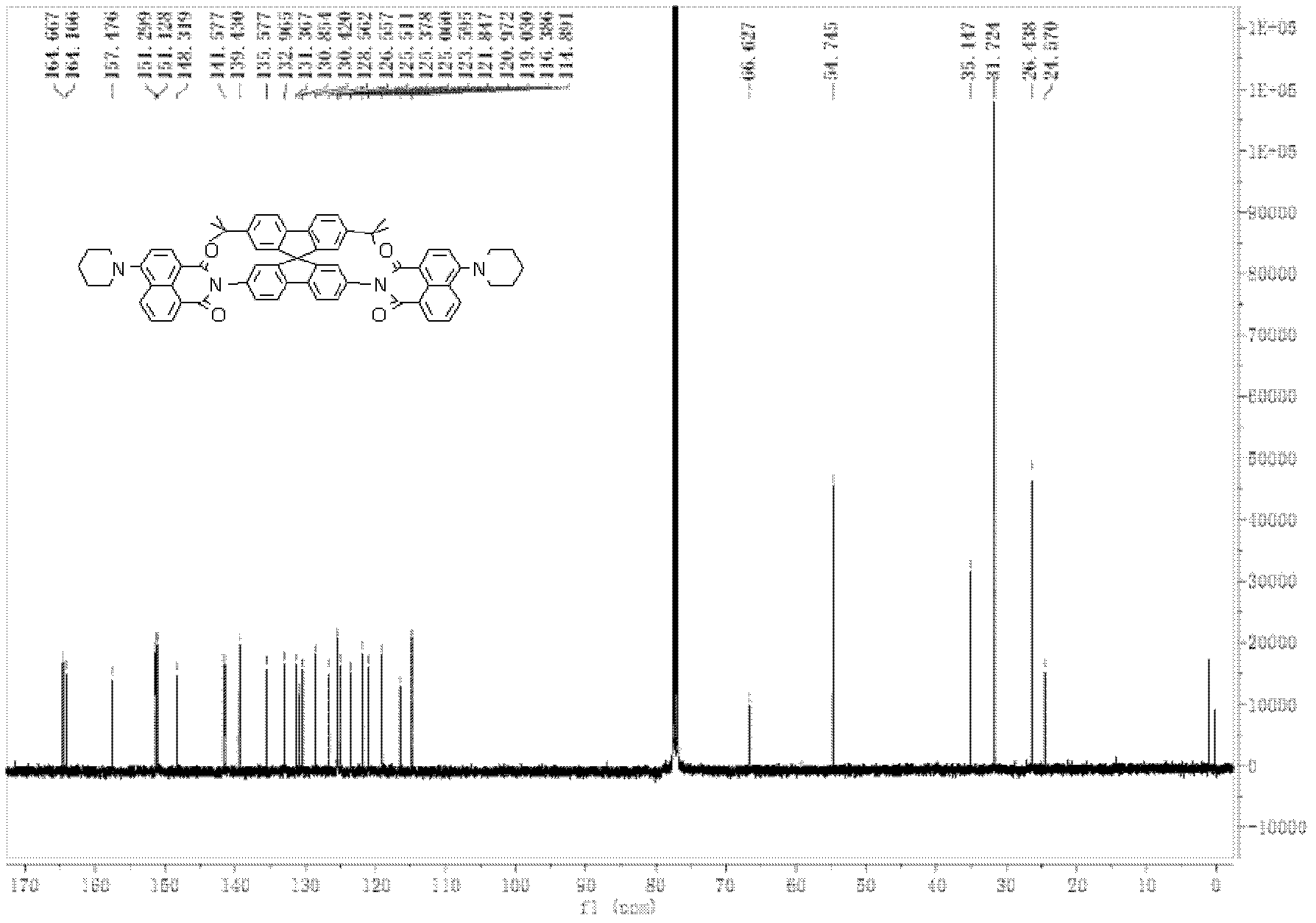
Top-bottom asymmetrical tert-butyl spirobifluorene compound
A tert-butylspirobifluorene, asymmetric technology, applied in the field of "up-down" asymmetric tert-butylspirobifluorene compound and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of 2,7-di-tert-butyl-2',7'-bis(4-piperidinenaphthalimide)-9,9'-spirobifluorene comprises the following steps:
[0094] (1) Synthesis of 2,7-di-tert-butyl-2',7'-dibromo-9,9'-spirobifluorene
[0095] Refer to the method disclosed in the prior Chinese patent CN 102126963 of the applicant.
[0096] (2) Synthesis of 2,7-di-tert-butyl-2',7'-bis(N-phthalimide)-9,9'-spirobifluorene
[0097]
[0098] Add 2g (3.4mmol) 2,7-di-tert-butyl-2',7'-dibromo-9,9'-spirobifluorene, 2.4g (12.97mmol) phthalimide to the three-necked flask Potassium, 3.2g (16.84mmol) ketone iodide, 30mL DMF, heated to reflux for 12h under the protection of nitrogen. The reaction mixture was cooled to room temperature, a large amount of turbidity appeared after adding concentrated ammonia water, suction filtered, and then extracted with dichloromethane (30mL×2), the solvent was evaporated, and the crude product was separated by column chromatography to obtain 1.342g of a yellow substance wit...
Embodiment 2
[0106] Determination of photoluminescence spectrum of "up-down" asymmetric tert-butylspirobifluorene compound (using 2,7-di-tert-butyl-2', 7'-bis(4-piperidine naphthalimide)- 9,9′-spirobifluorene as an example)
[0107] The product was formulated in solvents of different polarities to 1×10 -5 The solution of M was measured by absorption spectrum and emission spectrum in UV-Vis spectrometer and fluorescence spectrometer respectively. UV absorption spectrum see attached Figure 5 , the fluorescence emission spectrum is shown in the attached Image 6 . from Figure 5 It can be clearly seen that the maximum absorption spectrum of the compound is around 400nm and cuts off at 46nm, which fully meets the requirements of the transparency of nonlinear materials. from Image 6 It can be clearly seen that the maximum emission spectrum of the compound is around 500nm, and the maximum emission peak red shifts with the increase of solvent polarity. This shows that the charge in the m...
Embodiment 3
[0109] Thermal analysis of "up-down" asymmetric tert-butyl spirobifluorene compound (using 2,7-di-tert-butyl-2', 7'-bis(4-piperidine naphthalimide)-9, 9′-spirobifluorene as an example)
[0110] The product was thermally analyzed and tested on a Shimadzu DT-40 thermogravimetric analyzer. The operating conditions were: ceramic crucible (without cover), nitrogen atmosphere, air flow rate of 30mL / min, and heating rate of 10.0°C / min. The starting temperature is 25°C and the ending temperature is 800°C. The test results are attached Figure 7 . It can be observed from the figure that when the temperature reaches 385°C, the substance begins to decompose, and when it decomposes 5%, the temperature reaches 448°C. In the DTA curve, there is an obvious exothermic peak. At this time, the peak temperature is 369°C. This peak comes from the crystallization exotherm, and the temperature at this time is the crystallization temperature. After this temperature until 800 ° C, the system has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com