Method for synthesizing pyrene-4,5,9,10-tetralone
A technology for synthesizing pyrene and tetraketone, which is applied in chemical instruments and methods, preparation of organic compounds, carbon-based compounds, etc., can solve the problems of no production value, difficulty in raw material preparation, short steps, etc., and achieves mild conditions and synthesis Process-reliable, efficient oxidation reaction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for synthesizing pyrene-4,5,9,10-tetraketone is divided into two steps:
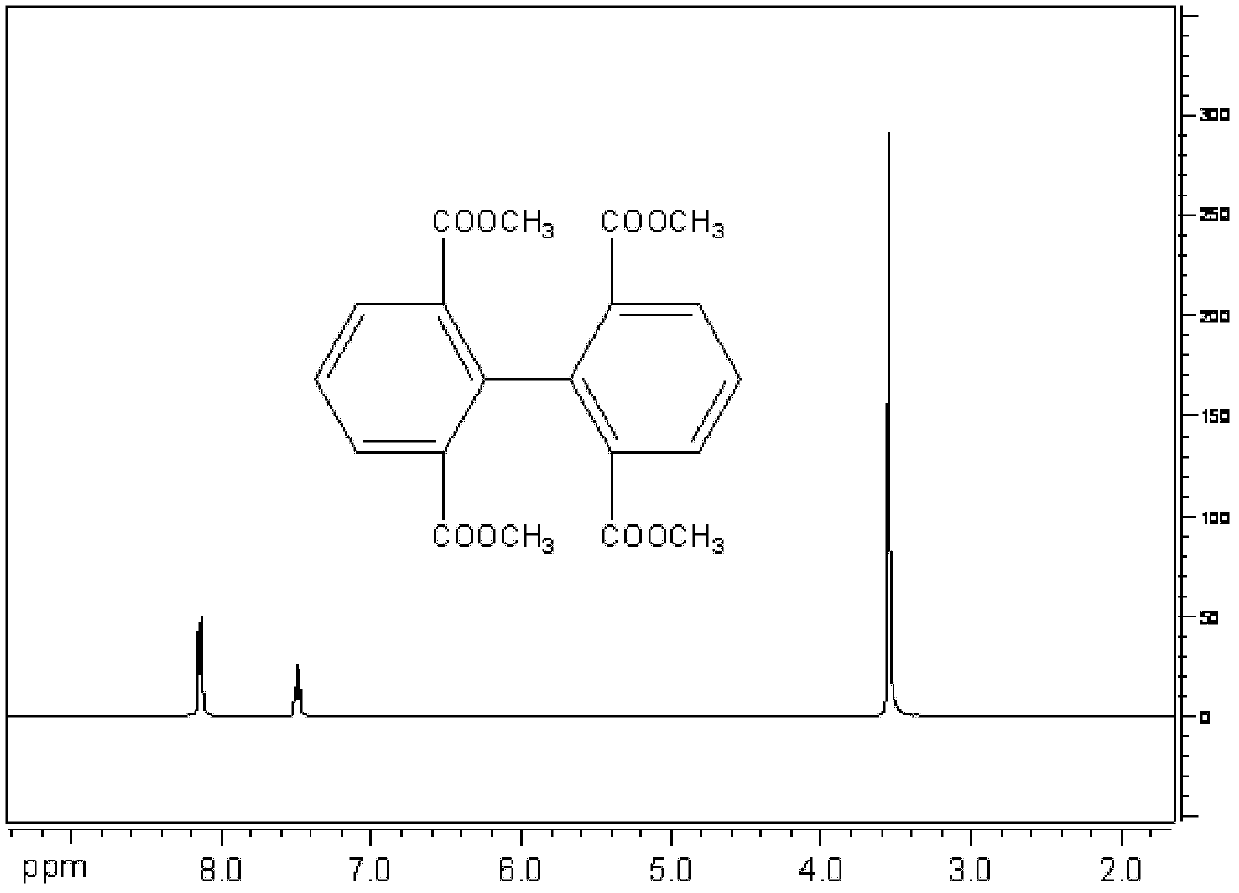
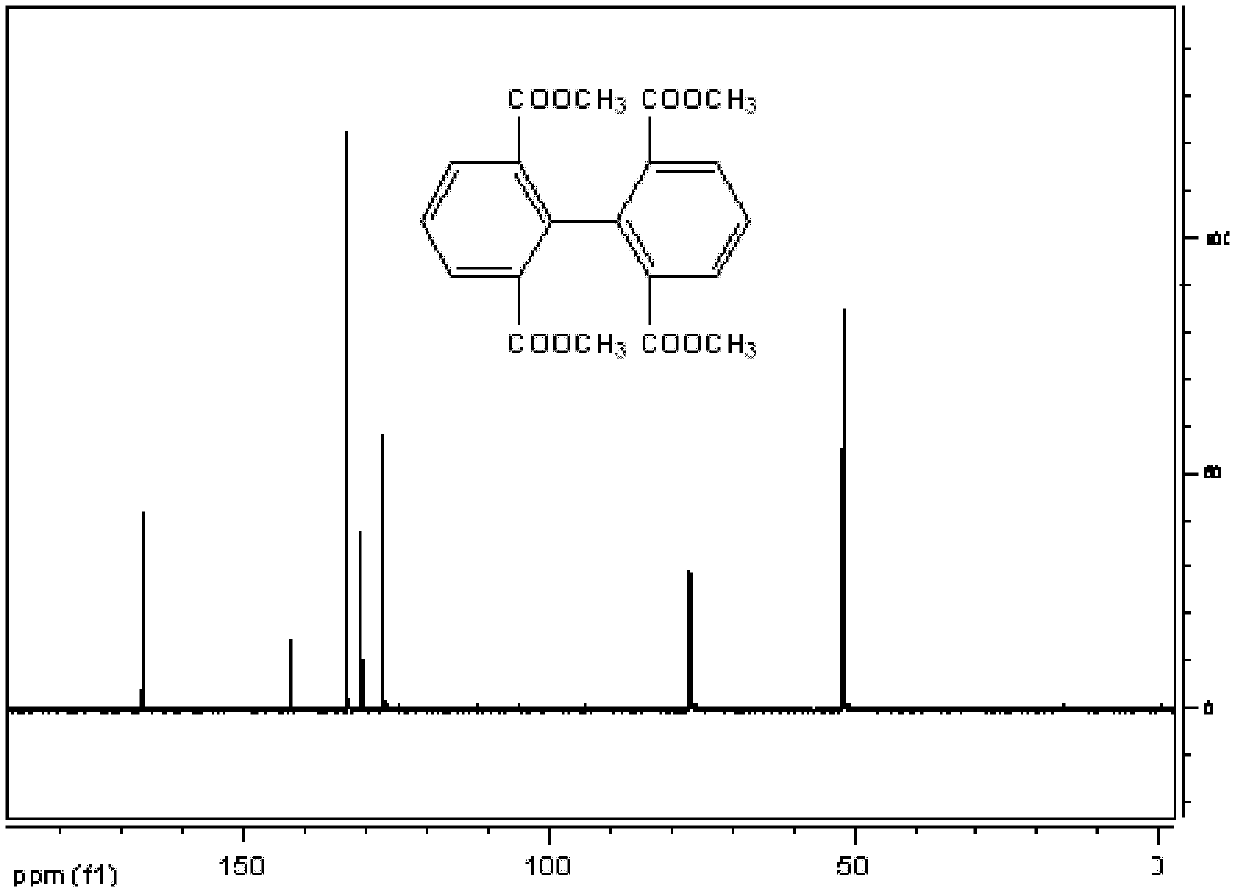
[0026] (1) Preparation of 2,2',6,6'-methyl-tetracarboxylate biphenyl
[0027] 103 grams of 2,6-dicarboxylic acid methyl ester-1-bromobenzene (purchased from aldrich company), 55 grams of copper powder, 180 milliliters of N, N-dimethylformamide (DMF) reacted for 6 hours at 125 degrees, cooling, Add 1000 ml of toluene, and filter off the copper powder. The filtrate was washed with plenty of water. Add anhydrous magnesium sulfate to dry. Filter and spin off toluene. A brown solid was obtained. Recrystallized with 1000 ml of ethanol to obtain 87 g of white powder with a yield of 78%. Melting point: 128-130°C. 1 H NMR (400MHz, CDCl 3 , ppm): 8.144 (d, J=8, 4H), 7.503 (t, J=8, 2H), 3.558 (s, 12H). 13 C NMR (100MHz, CDCl 3 ): 166.531, 142.319, 133.176, 130.676, 127.131, 52.042.
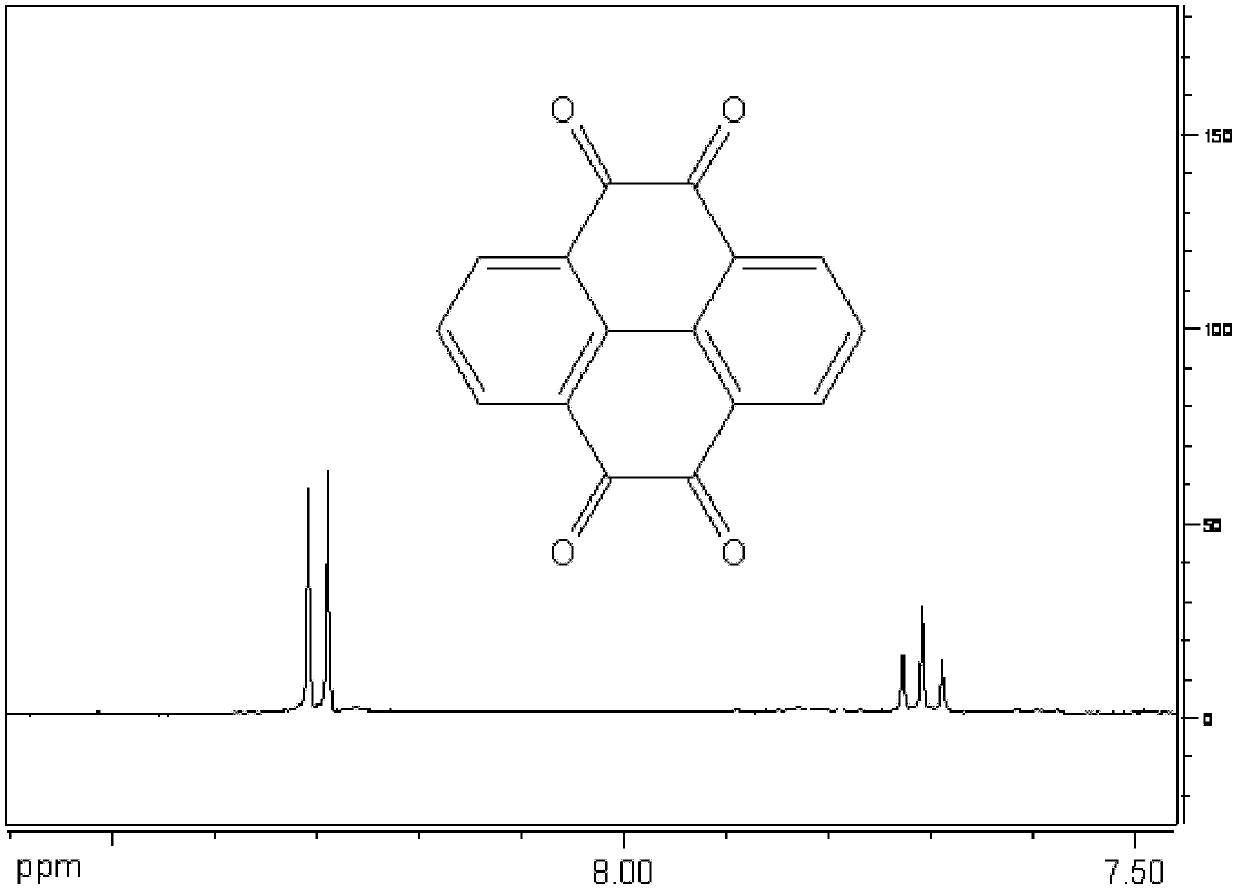
[0028] (2) Preparation of pyrene-4,5,9,10-tetraketone
[0029] Under a nitrogen atmosphere, 1.2 g of metal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com