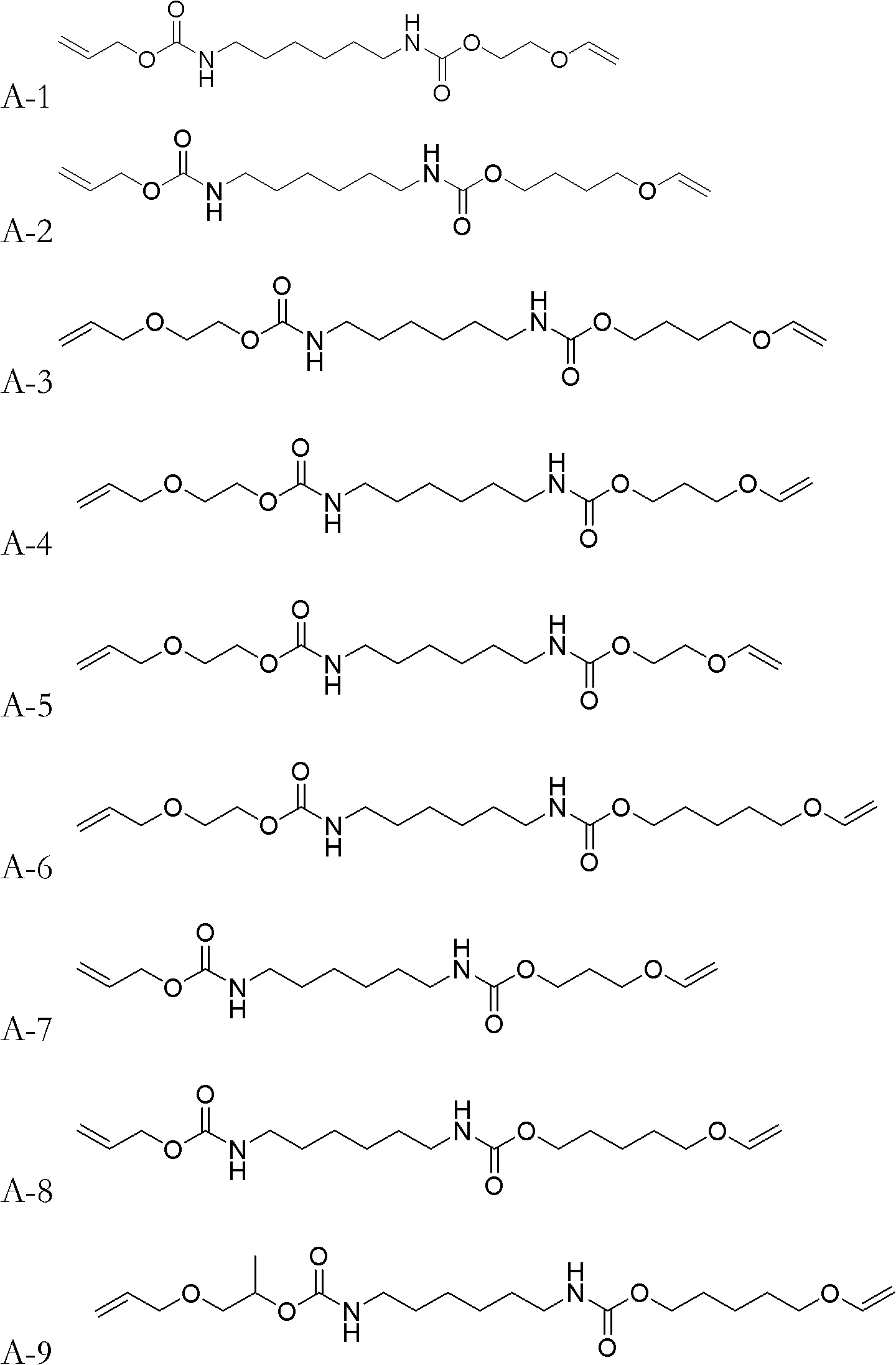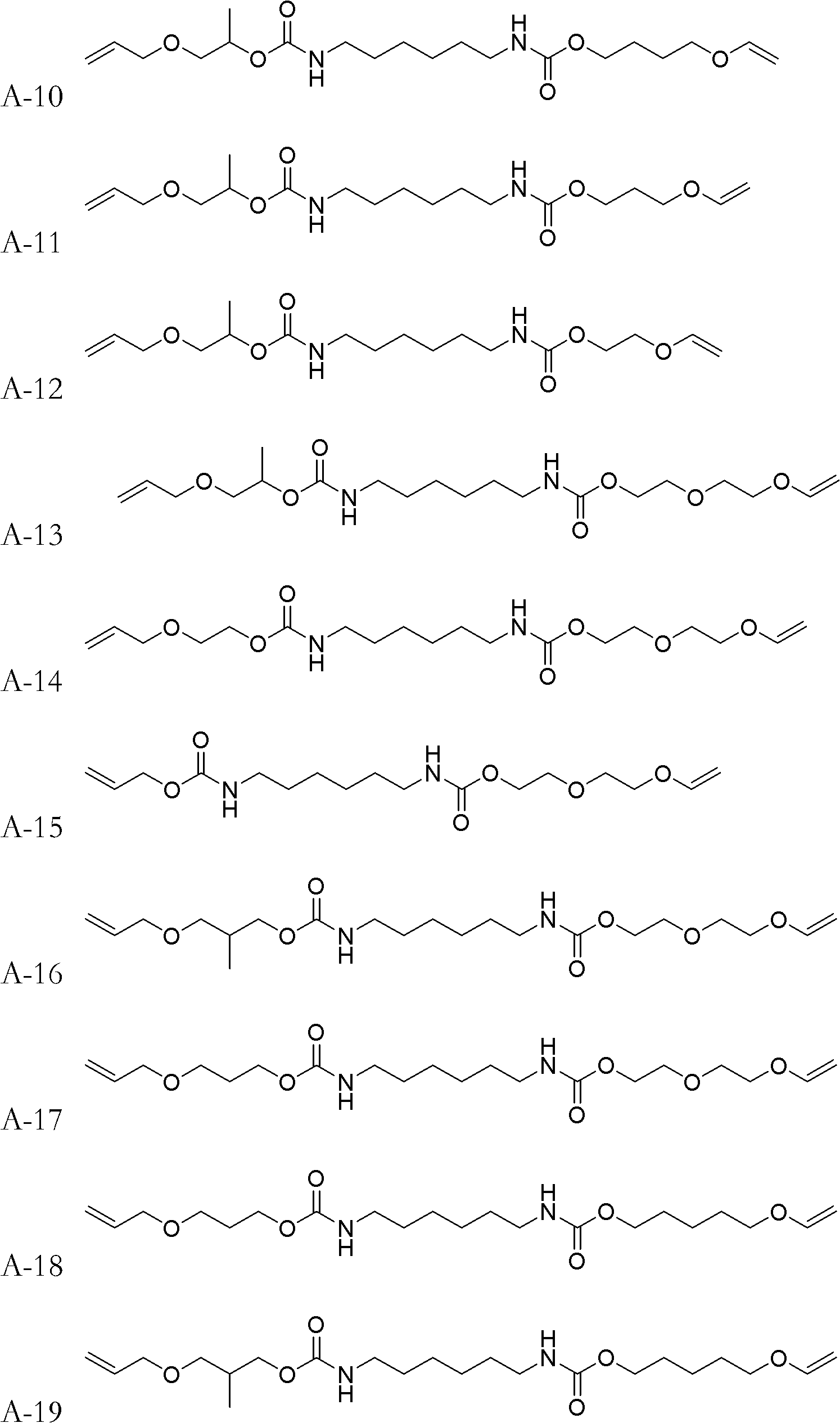Urethane polymeric monomer with end groups including vinyl ether and allyl ether and synthetic method thereof
A technology of vinyl ether and allyl ether, which is applied in the field of synthesis of urethane polymer monomers, can solve the problems of impact and moisture impact, and achieve the effects of accelerated polymerization rate, low cost, and convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
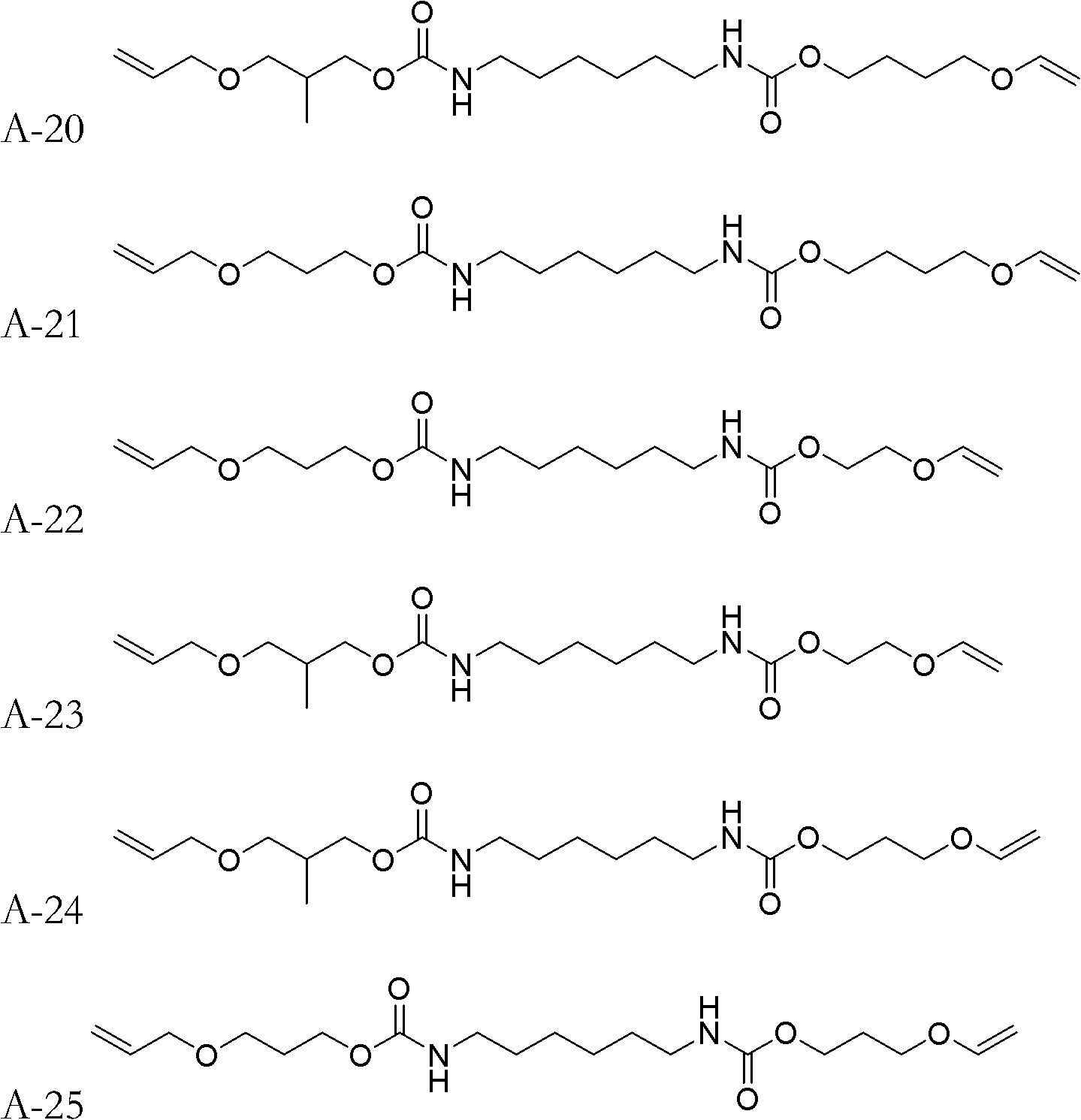
[0037] The synthetic method of monomer A-2:
[0038] Add 16.820g (0.10mol) of HDI (hexamethylene diisocyanate) and 0.2ml of dibutyltin dilaurate into a 500mL four-necked flask, then add 100ml of acetone solvent to dilute, and stir in an ice-water bath to cool it below 10°C . Dissolve 11.616g (0.10mol) of 4-hydroxybutyl vinyl ether in 50mL of acetone, and slowly add it dropwise into the four-necked flask, keeping the temperature of the solution in the four-necked flask below 10°C for about two hours Drip finished. After dropping, remove the ice-water bath, raise the temperature to 30°C and continue stirring for 1.5h, then add dropwise 50ml of acetone solution dissolved with 5.808g of allyl alcohol (0.10mol), and use the heat of reaction to maintain the temperature at about 20-40°C. After the dropwise addition was completed, the temperature was raised to 50-55° C. and the stirring reaction was continued for 2 h. After the reaction was completed, the acetone was removed by rot...
Embodiment 2
[0040] The synthetic method of monomer A-7:
[0041] Use 3-hydroxypropyl vinyl ether to replace 4-hydroxybutyl vinyl ether in [Example 1], and the remaining reagents and dosages are the same as [Example 1].
Embodiment 3
[0043] The synthetic method of monomer A-17:
[0044] Replace allyl alcohol in [Example 1] with 3-(allyloxy) propan-1-alcohol, and replace 4-hydroxybutyl vinyl ether in [Example 1] with diethylene glycol monovinyl ether , all the other reagents and consumption are identical with [Example 1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com