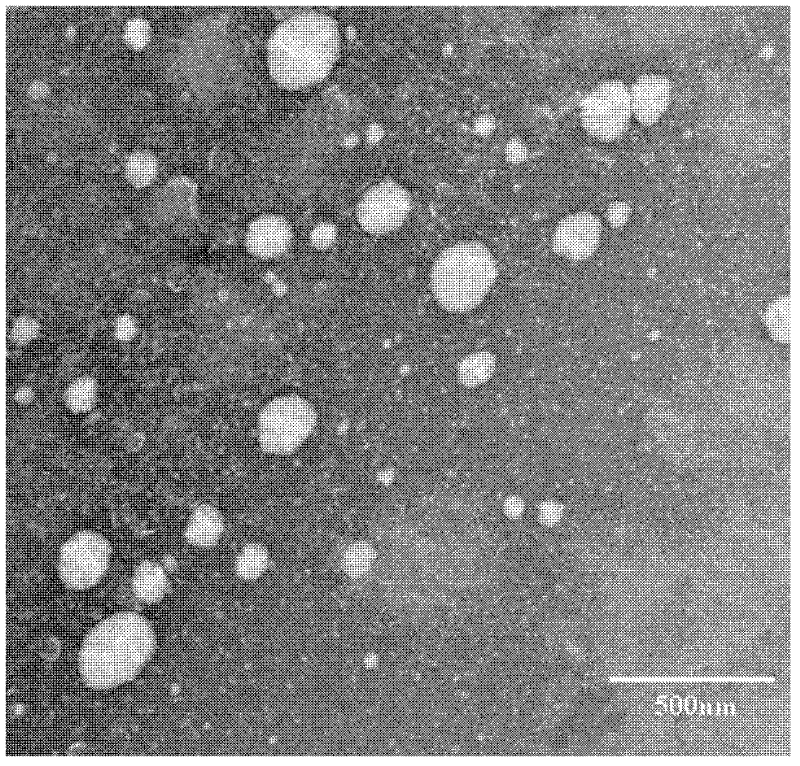
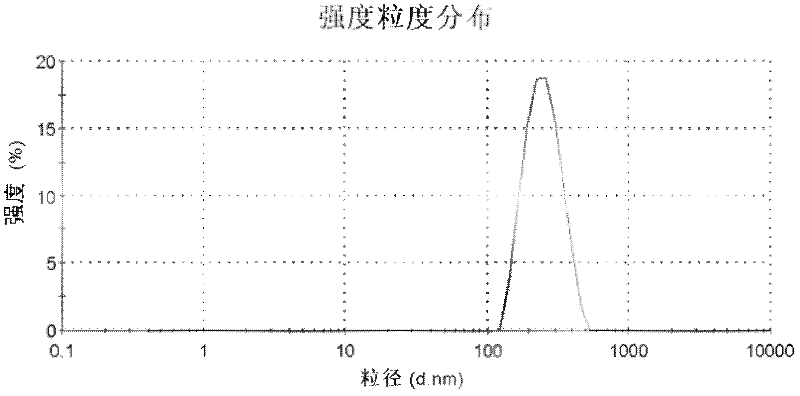
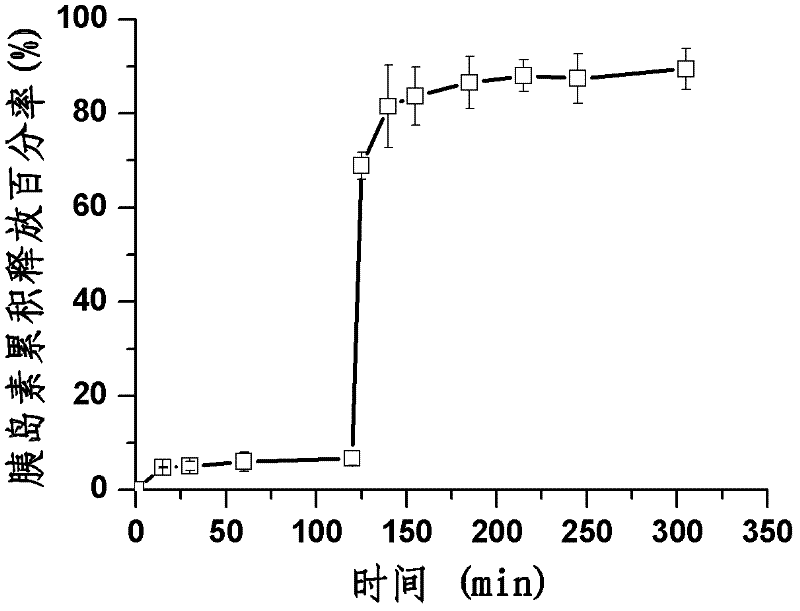
Preparation method of polypeptide/proteinic drug nanoparticle with high drug loading and high encapsulation efficiency
A nanoparticle and protein technology, applied to peptide/protein components, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as complex preparation methods, adverse effects on the health of operators and users, and low freezing points. The preparation process is simple, easy to scale up in industrial production, and the effect of a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of Insulin Hyaluronic Acid Nanoparticles
[0037] The formula is as follows:
[0038]
[0039] Preparation:
[0040] (1) Preparation of oil phase and water phase solutions
[0041] Weigh insulin and sodium hyaluronate into a vial, add ultrapure water to dissolve, then add Tween 80 as the water phase; weigh Span 80, put it into a 50ml centrifuge tube and dissolve it in cyclohexane as the oil phase; The phase is slowly dropped into the oil phase under the action of mechanical stirring (10000rpm, 3min) to obtain the drug nanoemulsion.
[0042] (2) Preparation of lyoprotectant emulsion
[0043] Weigh mannitol and dissolve it in ultrapure water, add Tween 80 as the water phase; weigh Span 80 and put it into a 50ml centrifuge tube, dissolve it in cyclohexane as the oil phase. An emulsion is obtained after mechanical dispersion.
[0044] (3) Preparation of protein nanoemulsion and lyoprotectant nanoemulsion mixture
[0045] Add the lyoprotectan...
Embodiment 2
[0049] Embodiment 2: Preparation of bovine serum albumin hyaluronic acid / alginic acid nanoparticles
[0050] The formula is as follows:
[0051]
[0052] Preparation:
[0053] (1) Preparation of oil phase and water phase solutions
[0054] Weigh bovine serum albumin, sodium hyaluronate, and alginic acid into a vial, add ultrapure water to dissolve, then add Tween 80 as the water phase; weigh lecithin into a 50ml centrifuge tube, dissolve in cyclohexane As the oil phase; the water phase is slowly dropped into the oil phase under the action of mechanical stirring (10000rpm, 3min) to obtain the drug nanoemulsion.
[0055] (2) Preparation of lyoprotectant emulsion
[0056] Weigh mannitol and dissolve it in ultrapure water, add Tween 80 as the water phase; weigh lecithin into a 50ml centrifuge tube, dissolve it in cyclohexane as the oil phase. A W / O emulsion is obtained after mechanical dispersion.
[0057] (3) Preparation of protein nanoemulsion and lyoprotectant emulsion m...
Embodiment 3
[0062] Example 3: Preparation of Insulin Hyaluronic Acid Nanoparticles
[0063] The formula is as follows:
[0064]
[0065] Preparation:
[0066] (1) Preparation of oil phase and water phase solutions
[0067] Weigh insulin and sodium hyaluronate into a vial, add ultrapure water to dissolve, add PEG400 monolaurate as the water phase; weigh Span 80, put it into a 50ml centrifuge tube and dissolve it in cyclohexane as the oil phase; Slowly drop the water phase into the oil phase under the action of mechanical stirring (10000rpm, 3min) to obtain the drug nanoemulsion.
[0068] (2) Preparation of lyoprotectant emulsion
[0069] Weigh sorbitol and dissolve it in ultrapure water, add PEG400 monolaurate as the water phase; weigh Span 80 and put it into a 50ml centrifuge tube, dissolve it in cyclohexane as the oil phase. A W / O emulsion is obtained after mechanical dispersion.
[0070] (3) Preparation of protein nanoemulsion and lyoprotectant emulsion mixture
[0071] Add th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com