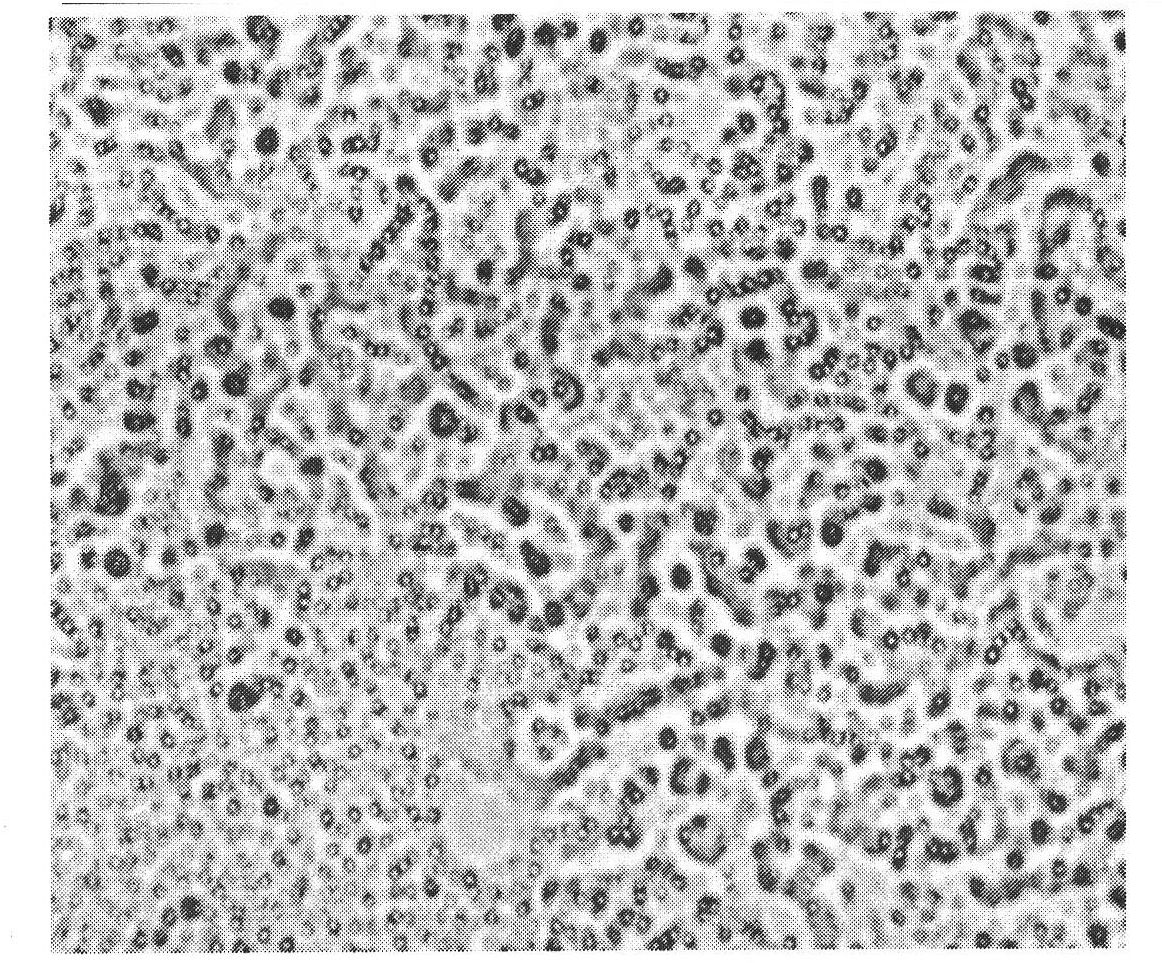
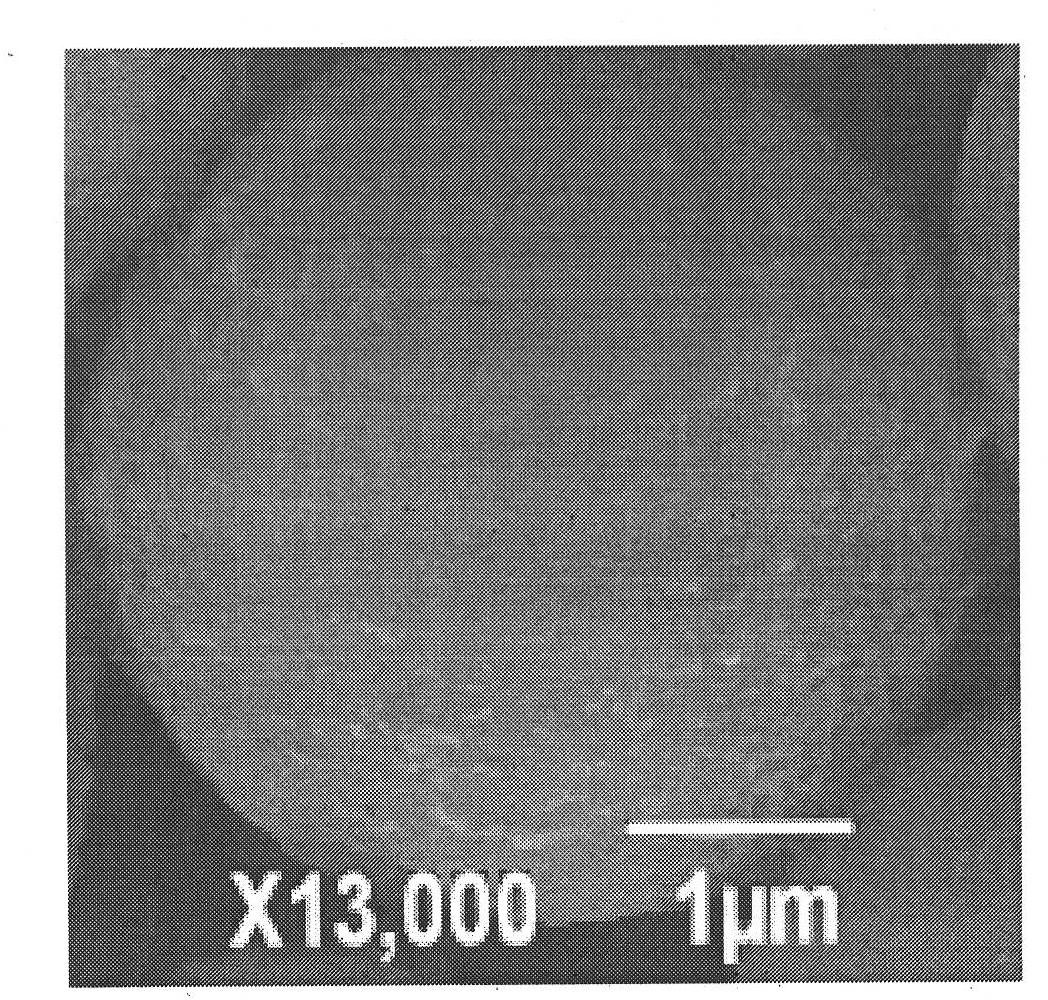
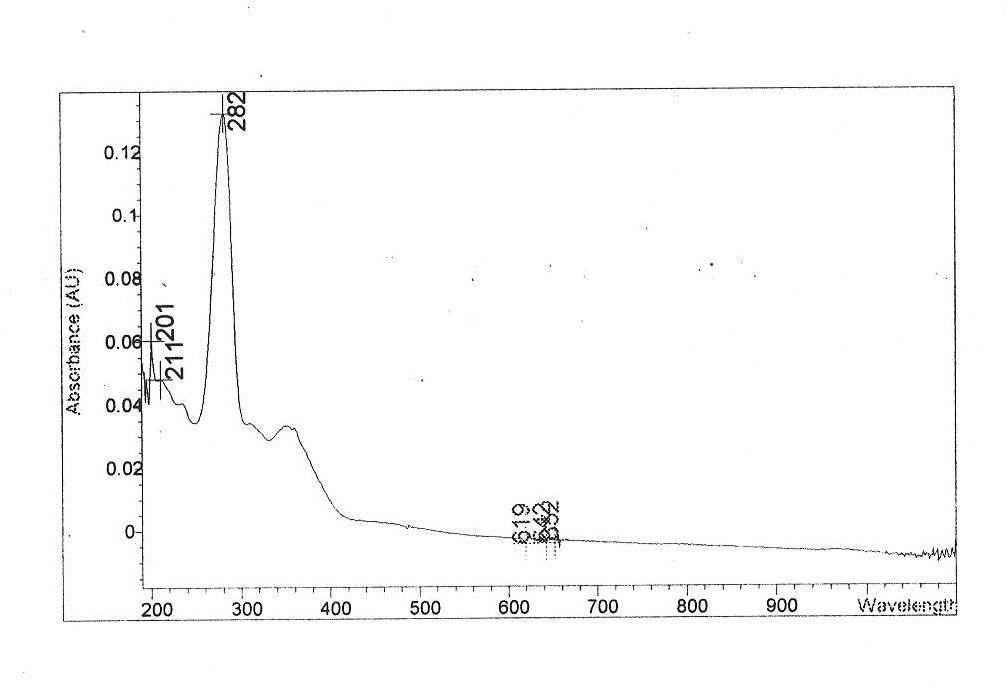
Veterinary danofloxacin mesylate slow-release microsphere preparation as well as preparation method and application of veterinary danofloxacin mesylate slow-release microsphere preparation
A technology of dafloxacin mesylate and slow-release microsphere preparations, applied in the field of veterinary dafloxacin mesylate slow-release microsphere preparations and its preparation, can solve the problem of low encapsulation rate of drug loading, toxicity Side effects, environmental pollution and other problems, to achieve the effect of long maintenance time of effective concentration, high drug loading, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of the present embodiment dafloxacin mesylate sustained-release microspheres is as follows:
[0043] (1) Accurately weigh 1.56 g of zinc acetate and 2.1 g of calcium chloride, and dissolve them in 35 mL of deionized water to obtain a cross-linking agent containing 6% calcium chloride and 4% zinc acetate.
[0044] (2) Accurately weigh 2.0 g of sodium alginate (Sigma-Aldrich, the same below), add 100 mL of deionized water, stir until completely dissolved, then add 0.2 g of sodium bisulfite, and magnetically stir until completely dissolved in a 50°C water bath dissolved to obtain a 2.0% sodium alginate solution.
[0045] (3) Accurately weigh 2.0 g of dafloxacin mesylate crude drug (purity 100.5%, purchased from Zhejiang Xinchang Guobang Veterinary Medicine Factory, the same below), dissolve it with 4-5 mL of deionized water, and add it to step (2) ) in the sodium alginate solution obtained, magnetically stirred under the condition of a water bath at...
Embodiment 2
[0054] The preparation method of the present embodiment dafloxacin mesylate sustained-release microspheres is as follows:
[0055] (1) Accurately weigh 1.56 g of zinc acetate and 2.8 g of calcium chloride, dissolve them in 35 mL of deionized water, and obtain a cross-linking agent containing 8% calcium chloride and 4% zinc acetate.
[0056] (2) Accurately weigh 2.0g of sodium alginate, add 100mL of deionized water, stir until completely dissolved, then add 0.2g of sodium bisulfite, stir magnetically in a water bath at 50°C until completely dissolved, and obtain 2.0% sodium alginate solution.
[0057] (3) Accurately weigh 2.0 g of dafloxacin mesylate crude drug with a purity of 100.5% (the source manufacturer is the same as in Example 1), dissolve it in 4 to 5 mL of deionized water, and add it to the sodium alginate solution obtained in (2). , 50 ° C under the condition of a water bath, magnetic stirring, so that it is evenly mixed to obtain an aqueous solution.
[0058] (4) ...
Embodiment 3
[0065] The preparation method of the present embodiment dafloxacin mesylate sustained-release microspheres is as follows:
[0066] (1) Accurately weigh 1.56 g of zinc acetate and 2.8 g of calcium chloride, dissolve them in 35 mL of deionized water, and obtain a cross-linking agent containing 8% calcium chloride and 4% zinc acetate.
[0067] (2) Accurately weigh 2.0g of sodium alginate, add 100mL of deionized water, stir until completely dissolved, then add 0.2g of sodium bisulfite, stir magnetically in a water bath at 50°C until completely dissolved, and obtain 2.0% sodium alginate solution.
[0068] (3) Accurately weigh 2.0 g of dafloxacin mesylate crude drug, dissolve it in 4-5 mL of deionized water, add it to the sodium alginate solution obtained in (2), and stir magnetically in a water bath at 50°C to mix Uniformly, an aqueous phase solution was obtained.
[0069] (4) Measure 100 mL of soybean oil for injection, add 5.0 g of span-80, and stir evenly to obtain an oil phas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com