Immunosorbent for blood purification and preparation method thereof
An immunosorbent and blood purification technology, applied in chemical instruments and methods, blood circulation treatment, other chemical processes, etc., can solve the problems of no immunosorbent, reduce treatment risk, reduce treatment cost, and reduce PRA level Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
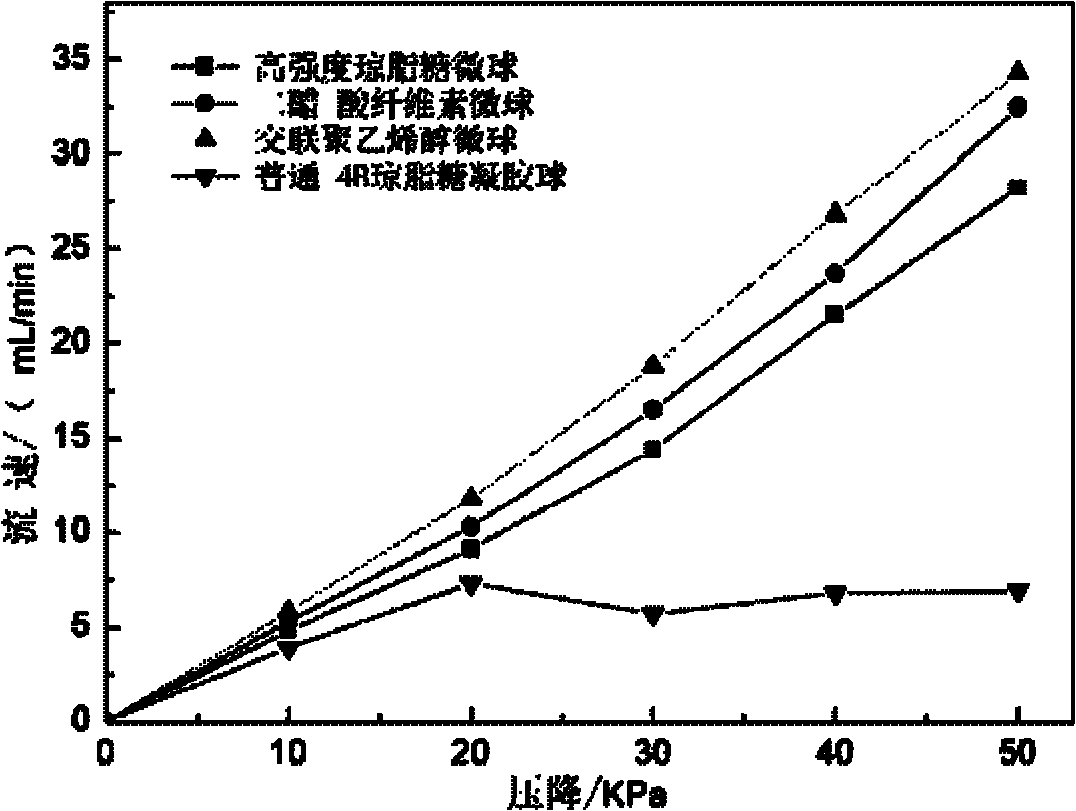
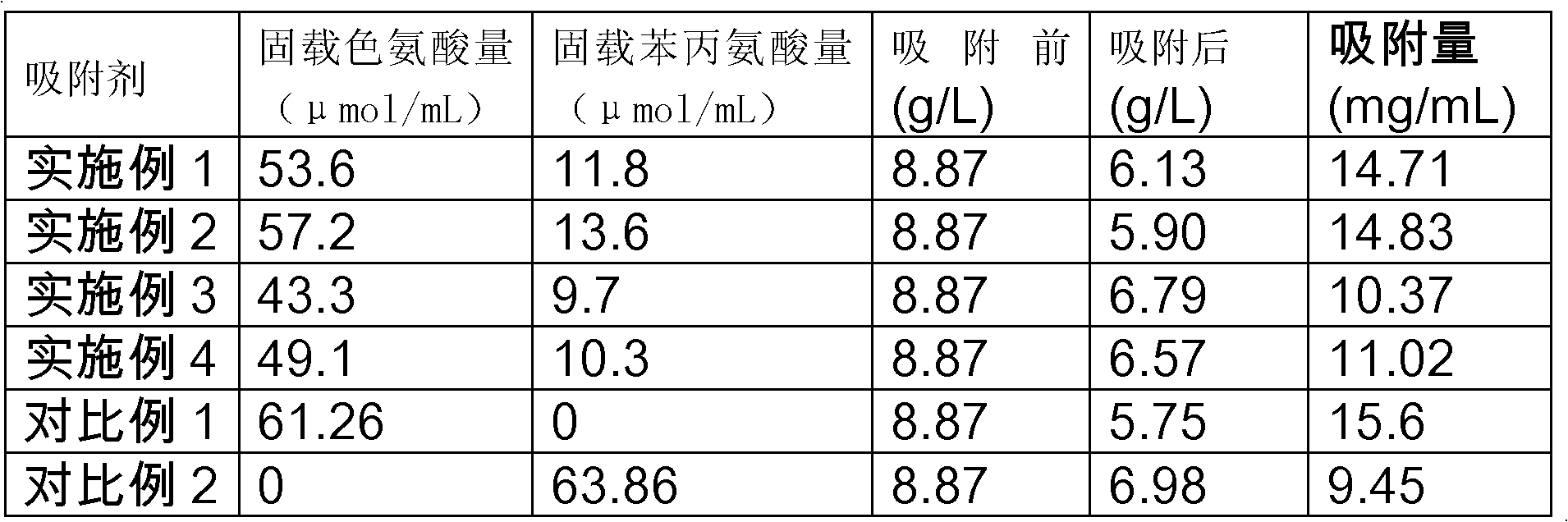
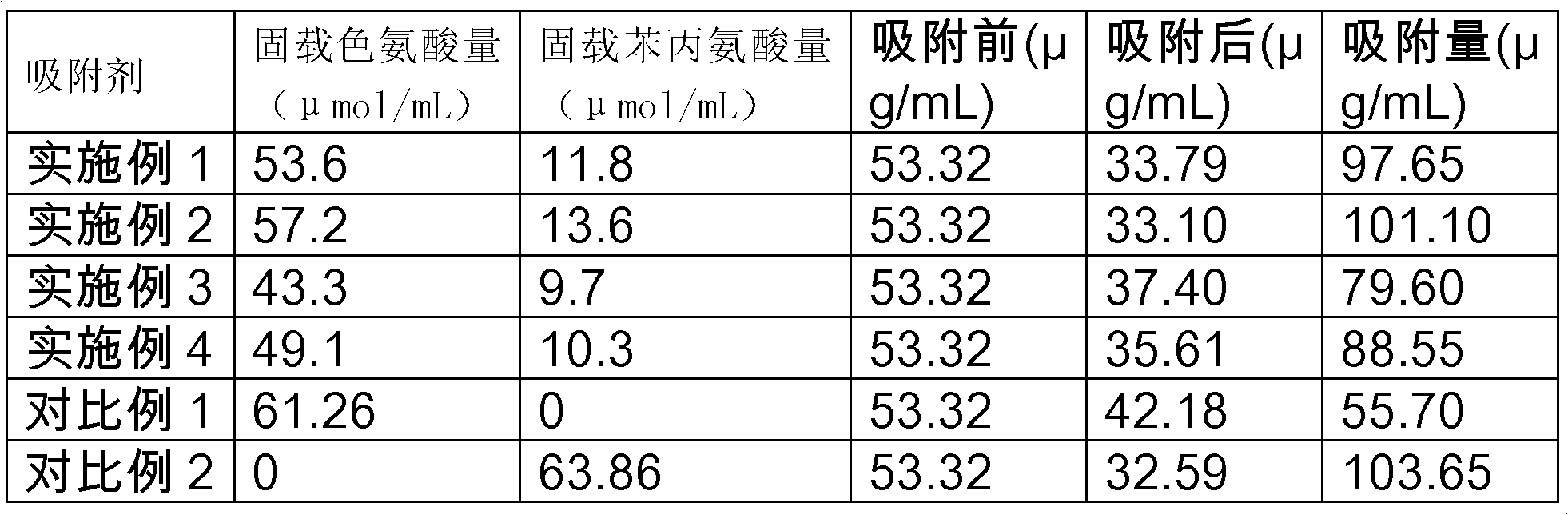
Image
Examples
Embodiment 1
[0043] Using high-strength agarose as a carrier, an immunoadsorbent for blood purification immobilized with a mixed amino acid ligand composed of tryptophan and phenylalanine amino acid, the preparation method comprises the following steps:
[0044] Step 1: Preparation of high-strength agarose support
[0045] Step 1-a: Preparation of agarose gel beads
[0046] Place a 500mL three-necked flask equipped with a stirring device in a 60°C water bath, and add 145mL of toluene, 55mL of chloroform, and 805mL of Tween into the bottle. Stir well and keep warm. Slowly pour 100 mL of the prepared agar solution with a mass fraction of 4% into a three-necked flask, and adjust the stirring speed to disperse the agar solution into droplets of appropriate size in the organic phase. After the dispersion is uniform (about 30min), remove the water bath, stir at room temperature and let the system cool down naturally. After the system was cooled, the organic solvent in the upper layer was disc...
Embodiment 2
[0057] The preparation method of this example is basically the same as that of Example 1, the only difference is that the immobilization process of the mixed amino acid ligands in step 3 is different. Since the preparation of the high-strength agarose carrier, the activation of epichlorohydrin, and the end-capping in this example are the same as those of steps 1, 2, and 4 in Example 1, for the sake of saving, it will not be repeated here, and the following is mainly Introduce Step 3 - Mixed Amino Ligand Immobilization Process.
[0058] Step 3: Immobilization of mixed amino acid ligands
[0059] Step 3-a, in 40mL of sodium phosphate buffer solution with a concentration of 0.2M and a pH value of 11, add 0.6g of tryptophan to prepare a tryptophan solution, and add 20mL of epoxy-activated agarose microspheres obtained in step c , reacted at 40° C. for 15 h, washed several times with sodium phosphate buffer solution with a pH value of 11 after the reaction, washed the unreacted tr...
Embodiment 3
[0063] Mixed Amino Acid Ligand Adsorbent Carrier of Cellulose Diacetate
[0064] Step 1: Preparation of cellulose diacetate carrier
[0065] Step 1-a: Preparation of cellulose diacetate gel beads
[0066] In a 500mL three-necked flask equipped with a stirring device, put 9.5g of cellulose diacetate and 100mL of dichloromethane into the solution, stir and dissolve, then add 25mL of dimethyl phthalate and 25mL of n-dodecyl alcohol as porogens. In another 1L three-necked flask equipped with a stirring device, first add 500 mL of 5% gelatin aqueous solution, and adjust a certain stirring speed. Slowly pour the above-prepared cellulose diacetate solution into the gelatin solution. After the oil phase is dispersed and stabilized in the water phase, keep the stirring speed constant, fill the three-necked bottle with nitrogen, and heat it to 45°C. After the methyl chloride is volatilized, the resulting cellulose balls are sieved to collect 40-50 mesh gel balls, which are extracted w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com