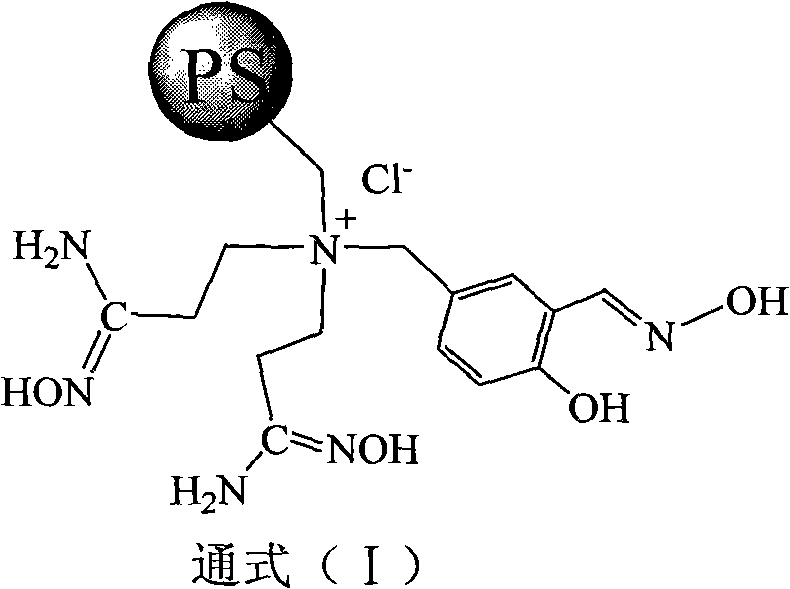
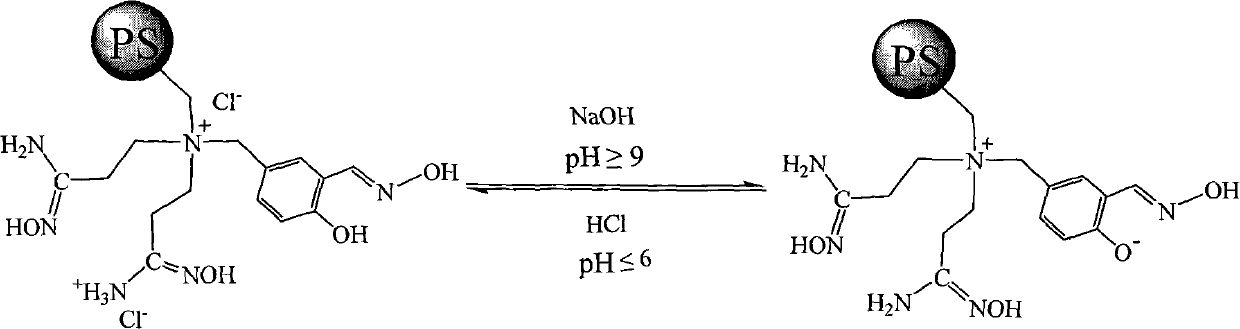
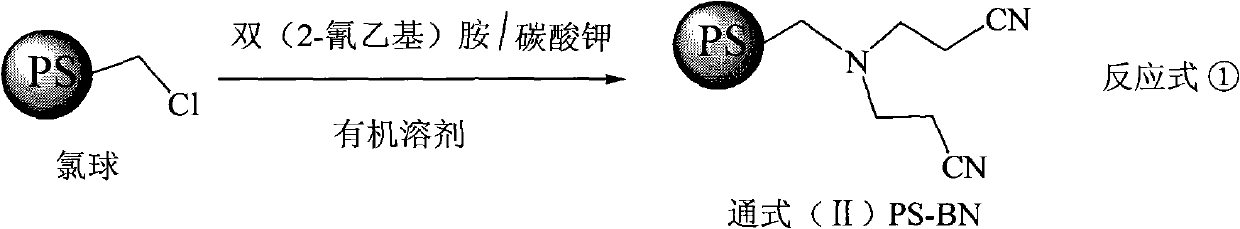
Super chelate type ion exchange resin, preparation method thereof, and application thereof
A technology for chelating ions and exchange resins, which is applied in the field of functional polymer materials and chelating ion exchange resins, and can solve the problems of no separation, enrichment, recovery of metal ions, complex chemical structures, and high synthesis costs. Achieve the effects of simple and easy preparation technology, high yield, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation and characteristics and application of embodiment 1 PS-BNSH-1 super chelating ion exchange resin
[0027] Step 1 Preparation of PS-BN-1
[0028]
[0029] Take by weighing commercially available cross-linking degree 4 polystyrene porous pellets and 180 grams of 1,4-dioxane with a chlorine content of 14.7%, and continuously drop 200 grams of bis(2-cyanoethyl Base) amine, after stirring and reacting for 60 hours, add 80 grams of sodium carbonate, continue to stir for 1 hour. The pellets were filtered out from the reaction system, washed three times with deionized water, and dried to obtain 143.6 g of PS-BN-1. Elemental analysis: nitrogen content 10.35%.
[0030] Step 2 Preparation of PS-BNS-1
[0031]
[0032] 80 g of 5-chloromethyl salicylaldehyde was weighed and dissolved in 400 ml of ethyl acetate, and the PS-BN-1 obtained in Step 1 was put into the reaction system, and stirred at room temperature for 48 hours. The pellets were taken out by filtrat...
Embodiment 2
[0037] Preparation and Application of Example 2 PS-BNSH-2 Super Chelating Ion Exchange Resin
[0038] According to the method and operation steps of Example 1, the polystyrene porous beads with a degree of crosslinking of 4 and a chlorine content of 14.7% in Step 2 of Example 1 were replaced with polystyrene porous beads with a degree of crosslinking of 2 and a chlorine content of 17.3%. Polystyrene porous bead, promptly makes PS-BNSH-2 super chelating ion exchange resin, after analyzing, it is known that PS-BNSH super chelating ion exchange resin is to Zn 2+ The saturated adsorption capacity is 7.2mmol / g, for Cu 2+ The saturated adsorption capacity was 7.2 mmol / g.
Embodiment 3
[0039] Example 3 The influence of pH on the adsorption capacity of PS-BNSH-1 multifunctional polystyrene chelating resin
[0040] Prepare 5000 milliliters of 50.00mg / L zinc chloride aqueous solution, use the hydrochloric acid of 20% by mass percentage and 30% sodium carbonate aqueous solution to adjust the pH of zinc chloride aqueous solution=2, 3, 4, 5, 6, 7, 8 respectively , 9, the ion-exchange column that 100 grams of PS-BNSH-1 super chelating ion-exchange resins are housed in continuous rinsing was used for 2 hours, and the Zn in the zinc chloride aqueous solution was measured using a flame atomic absorption spectrometer 2+ The concentration change, calculate the amount of the adsorbed zinc ion of PS-BNSH-1 super chelating ion exchange resin (in the millimole of zinc ion adsorbed per gram of resin), the results are shown in Table 1.
[0041] Table 1 pH and PS-BNSH-1 adsorption of Zn 2+ Relationship
[0042]
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com