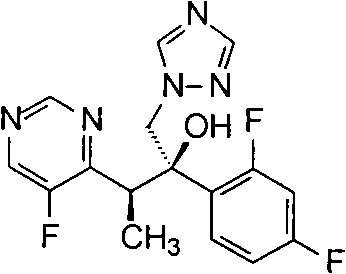
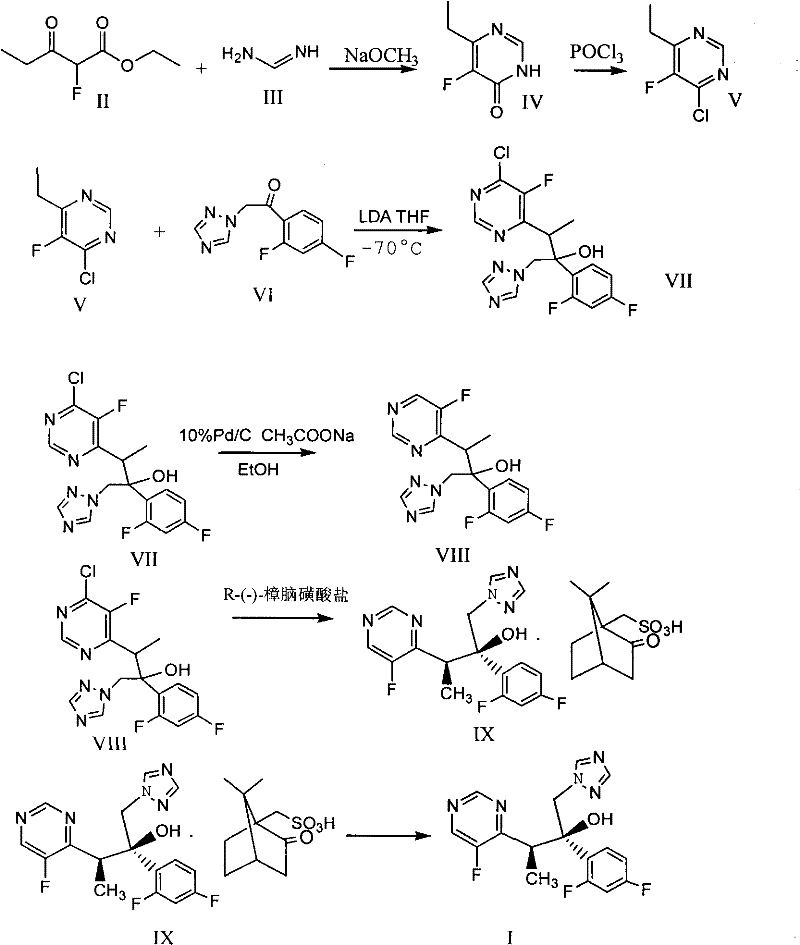
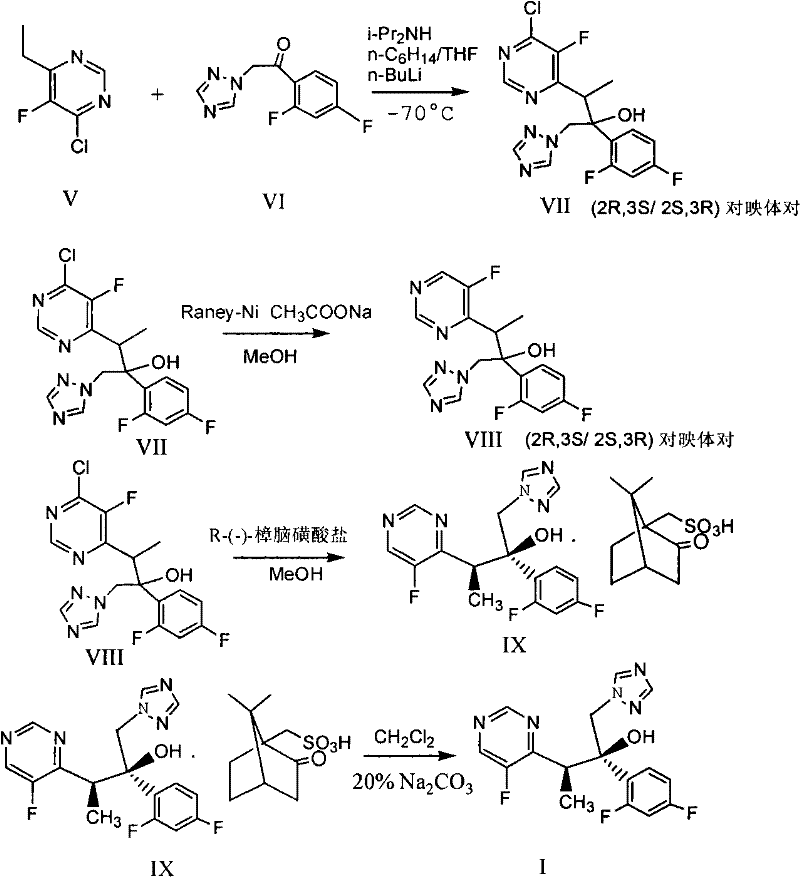
Novel method for producing voriconazole
A technology of voriconazole and fluoropyrimidine, applied in the field of voriconazole, can solve the problems of poor safety, unfavorable industrial production, many steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1 (2R, 3S)-3-(5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl-1-(1H-1,2,4-triazole-1- Base)-2-Butanol R-(-)-Camphorsulfonate Preparation
[0096] In the presence of nitrogen, 37.9 grams of zinc powder and 1.89 grams of lead powder were added to 180 ml of tetrahydrofuran solution, heated to reflux for 3 hours, cooled to room temperature, and 100 ml of tetrahydrofuran solution containing 29.4 grams of iodine was added dropwise within 20 minutes, and the temperature was raised to 45°C, after the addition, cool to -5 to 0°C, dropwise add 25.9 g of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl) 110 ml of a tetrahydrofuran solution of acetone and 110 ml of a tetrahydrofuran solution containing 27.8 grams of 4-chloro-6-(1-bromoethyl)-5-fluoropyrimidine, kept the temperature below 5°C during the addition, and stirred at 0 to 10°C for 3 Hours, raised to room temperature, left overnight; kept at 25 ° C, within 30 minutes, dropwise added an acid solution consisting of 34....
Embodiment 2
[0097] Example 2 (2R, 3S)-3-(5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1 Preparation of -yl)-2-butanol R-(-)-camphorsulfonate
[0098] In the presence of nitrogen, add 37.9 grams of zinc powder and 1.89 grams of lead powder to 180 ml of tetrahydrofuran solution, heat to reflux for 3 hours, cool to room temperature, and within 20 minutes, add 100 ml of tetrahydrofuran solution containing 29.4 grams of iodine, and heat up to 45 ° C during the addition process , Cool to -5 to 0°C after addition, dropwise add 25.9 grams of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone 110ml of tetrahydrofuran solution and 110ml of tetrahydrofuran solution containing 27.7 grams of 4-chloro-6-(1-bromoethyl)-5-fluoropyrimidine, keep the temperature below 5°C during the addition process, and stir at 0 to 10°C for 3 hours, Warm to room temperature and let stand overnight. Keep at 25°C, within 30 minutes, add dropwise an acid solution consisting of 57.2 grams of co...
Embodiment 3
[0099] Example 3 (2R, 3S)-3-(-5-fluoropyrimidin-4-yl-2-(2,4-difluorophenyl-1-(1H-1,2,4-triazole-1- base)-2-butanol preparation
[0100](2R,3S)-3-(-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl-1-(1H-1,2,4-triazol-1-yl) 19.1 g of -2-butanol R-(-)-camphorsulfonate was dissolved in a solution composed of 92 ml of dichloromethane and 100 ml of water, and the pH value was adjusted to about 11 with 40% NaOH solution, and the solution was layered and separated. The aqueous phase was extracted with 25ml of dichloromethane, and the organic layers were combined, washed with water 3×100ml, concentrated under reduced pressure and evaporated to remove the solvent. Add 30ml of isopropanol, heat to 45°C, evaporate 8ml of isopropanol under reduced pressure, and cool to 0°C, stirred for 1 hour, added a little seed crystal, filtered the precipitated solid, washed the solid with 5ml of cooled isopropanol, and dried to obtain 10.5 g of white solid, yield 91.5%, melting point 126-127°C, optical p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com