Water-soluble triethanolamine derivative
A technology of compound and propylamine, which is applied in the fields of drug combination, anesthetic, organic chemistry, etc., can solve the problems of prolonging the time of anesthesia, and achieve the effect of avoiding injection pain and embolism, facilitating absorption, and good anesthesia and analgesia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
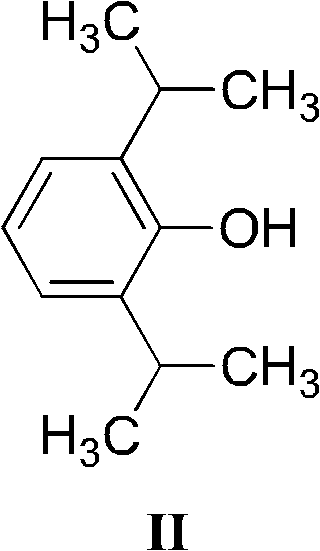
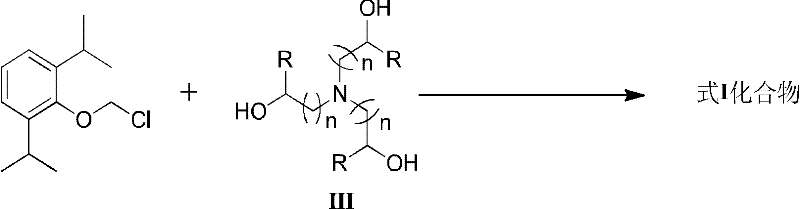
[0023] Embodiment 1: Preparation of Propofol Chloromethyl Ether
[0024]
[0025] Under argon protection, 10.0 g of propofol was added to a stirred suspension of sodium hydride (2.2 g, 60% dispersion in mineral oil) in anhydrous dimethoxyethane, and the resulting mixture was continuously Stir for 15 minutes, then in 15 minutes, in the homogeneous solution that forms, dropwise add chloroiodomethane 12.3ml, filter after stirring for 3 hours, evaporate solvent and excess chloroiodomethane, residue uses silica gel column chromatography ( Petroleum ether: ethyl acetate = 10:1 (v:v)) to obtain 10.2 g of a white solid.
[0026] Yield: 80.1%, purity: 99%.
[0027] 1 H NMR (300 MHz, CDCl3) δ: 7.08-7.20 (m, 3H), 5.75 (s, 2H), 3.36 (m, 2H), 1.21 (d, 12H).
Embodiment 2
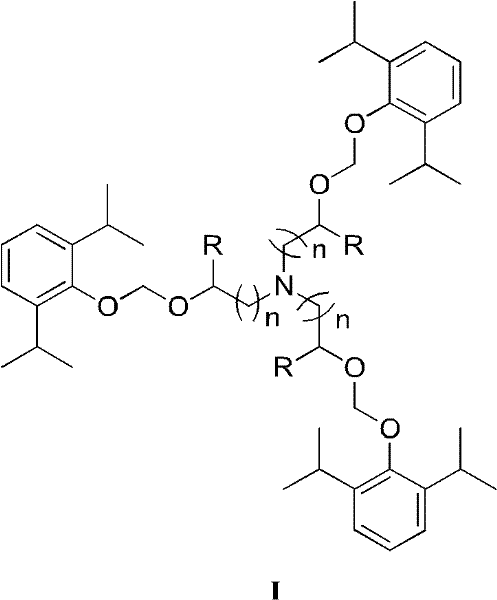
[0028] Embodiment 2: the preparation of three-2-(propofol O-methoxy)ethylamine
[0029]
[0030] Under the protection of nitrogen, add 2.65g sodium hydride (60%) in batches to 50ml tetrahydrofuran solution of 2.0g triethanolamine at 0°C, after stirring for 15 minutes, add dropwise 50ml of tetrahydrofuran solution of 5.0g propofol chloromethyl ether, about After 20 minutes of dripping, heat up to 20°C and stir for 3 hours, cool in an ice bath, add 2ml of water dropwise, concentrate under reduced pressure for 0.5 hours to exhaust the solvent (-90KPa, 35°C), and purify the remaining oil by silica gel column chromatography (petroleum ether : ethyl acetate=5:1 (v:v)) to obtain 7.8 g of white solid.
[0031] Yield: 82.0%, purity greater than 99%.
[0032] 1 H NMR (300MHz, DMSO-d6) δ: 7.10-7.21 (m, 9H), 5.92 (s, 6H), 3.95 (t, 6H), 3.40 (m, 6H), 2.61 (t, 6H), 1.25 ( d, 36H).
Embodiment 3
[0033] Embodiment 3: the preparation of three-2-(propofol O-methoxyl) propylamine
[0034]
[0035] According to the method of Example 2, tri-2-(propofol O-methoxy)propylamine can be prepared with propofol chloromethyl ether and triisopropanolamine under the action of sodium hydride.
[0036] 1 HNMR (300MHz, DMSO-d6) δ: 7.10-7.21 (m, 9H), 5.93 (s, 6H), 4.10 (m, 3H), 3.40 (m, 6H.), 2.52-2.76 (m, 6H), 1.26(d, 36H), 1.15(d, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com