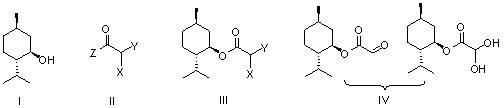
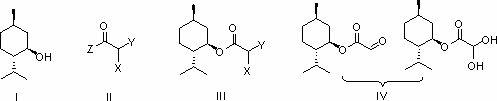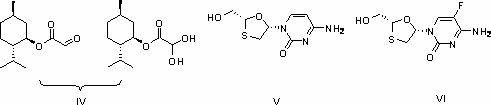Preparation methods of glyoxylic acid L-menthyl alcohol ester and monohydrate of glyoxylic acid L-menthyl alcohol ester
A technology of menthol esters and glyoxylic acid, applied in the preparation of carboxylic acid halides, organic chemistry, etc., can solve the problems of explosion safety hazards, high prices, and ineffectiveness, and achieve mild reaction conditions, less reaction side reactions, and excellent operating techniques easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of L-menthyl monochloroacetate (Ⅲ)
[0036] Add L-menthol (15.6g, 0.1mol), pyridine (8.6g, 0.11mol) and dichloromethane (200mL) to the reaction flask equipped with a stirrer, start stirring and cool with ice-salt bath, and add to the reaction flask A solution of chloroacetyl chloride (12.4g, 0.11mol) in dichloromethane (50mL) was slowly added dropwise to the bottle, and the drop was completed within 2 hours. The menthol disappeared, and the obtained white suspension was filtered, and the filtrate was washed with dilute hydrochloric acid (80mL, 2N), saturated aqueous sodium bicarbonate solution (80mL) and saturated brine (100mL), then dried over anhydrous sodium sulfate, and finally evaporated. The solvent dichloromethane was removed to obtain 22.3 g of L-menthyl chloroacetate as a colorless transparent liquid, with a yield of 95%.
Embodiment 2
[0037] Embodiment 2: Preparation of L-menthyl monobromoacetate (Ⅲ)
[0038] Add L-menthol (15.6g, 0.1mol), pyridine (8.2g, 0.105mol) and dichloromethane (200mL) into the reaction flask equipped with a stirrer, start stirring and cool the reaction system in an ice-salt bath to 0 o C. Slowly add a solution of bromoacetyl bromide (22g, 0.11mol) in dichloromethane (50mL) dropwise to the reaction flask, drop it over within 2 hours, then place the reaction system at room temperature for 1.5 hours, monitor with TLC until The raw material L-menthol disappeared, and the obtained white suspension was filtered, and the filtrate was washed with dilute hydrochloric acid (80mL, 2N), saturated aqueous sodium bicarbonate solution (80mL) and saturated brine (100mL), and then dried over anhydrous sodium sulfate , Distilled off the solvent methylene chloride to obtain 26.7 g of L-menthyl bromoacetate as a colorless transparent liquid, with a yield of 96%.
Embodiment 3
[0039] Example 3: Preparation of L-menthyl chloroacetate (Ⅲ) from chloroacetic anhydride
[0040] Add L-menthol (15.6g, 0.1mol), pyridine (8.6g, 0.11mol) and dichloromethane (200mL) to the reaction flask equipped with a stirrer, and then add chloroacetic anhydride (25.7g, 0.15 mol) was slowly added, stirred at room temperature until the raw material L-menthol disappeared, then the solvent was removed, dichloromethane was added to the obtained oil, washed with saturated aqueous sodium bicarbonate (80mL) and saturated brine (100mL), organic The phase was dried by adding anhydrous sodium sulfate, the desiccant was filtered off, and the solvent was evaporated to obtain 21.0 g of L-menthyl chloroacetate with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com