Hydrochloric acid boningmycin solid preparation and preparation method thereof
A technology of boningmycin hydrochloride and solid preparations, applied in the field of medicine, can solve problems such as poor stability, achieve good stability, simple preparation process, simple production conditions and equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
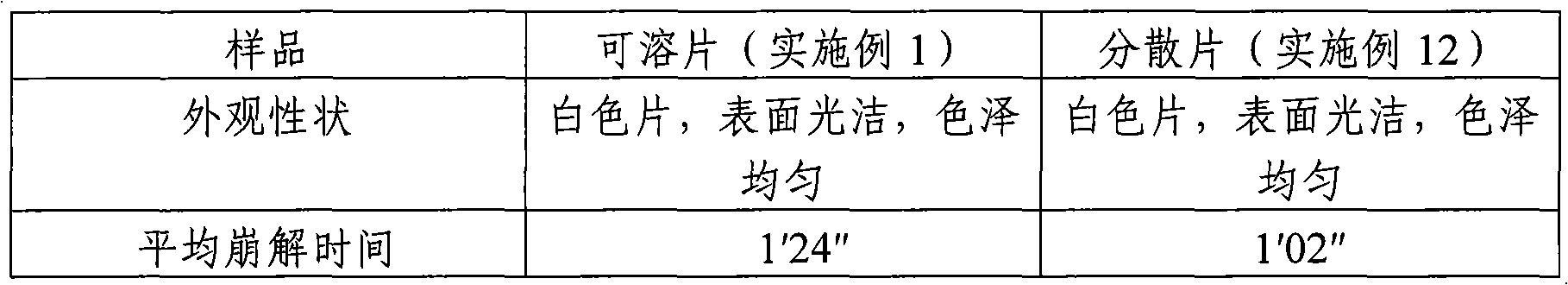
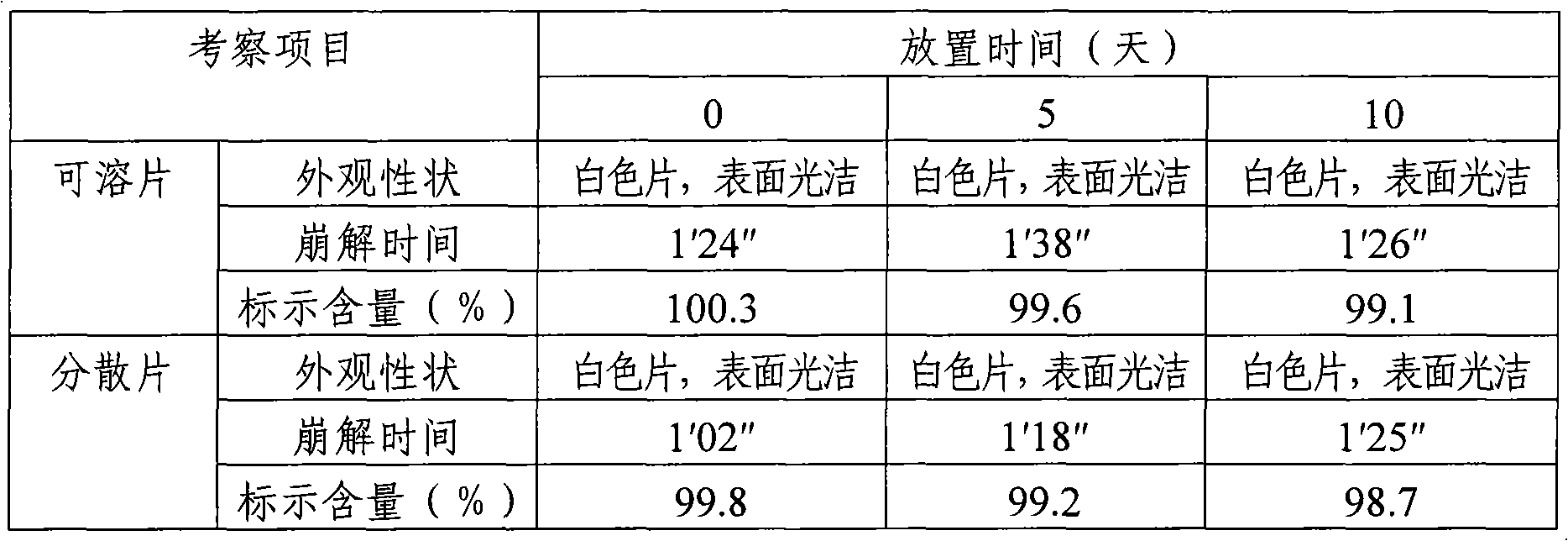
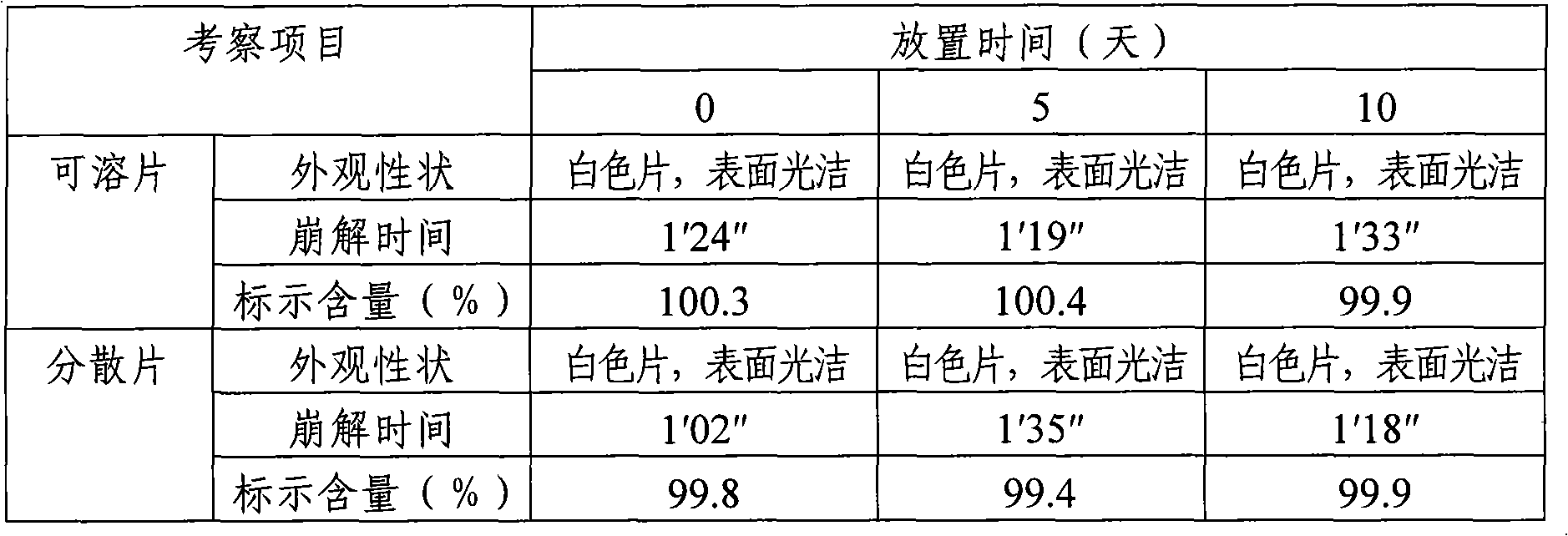
[0026] Calculated by preparing 1000 tablets, the weight of raw and auxiliary materials consists of: boningmycin hydrochloride 15g, lactose 251g as filler, polyethylene glycol 4000 3g as lubricant, polyvinylpyrrolidone K30 6g as binder, 25g of sodium carboxymethyl starch as a disintegrant. Mix the raw materials and auxiliary materials of the above-mentioned prescription amount evenly, and directly compress into tablets to obtain the bonomycin hydrochloride soluble tablet of the present invention, each tablet contains 15 mg of bonomycin hydrochloride, and the theoretical tablet weighs 300 mg.
[0027] Preparation of special solvent for soluble tablets (dispersible tablets): Take 20g of hypromellose 2910, 1g of benzalkonium chloride, add an appropriate amount (about 300ml) of hot water at 80-90°C, stir well to hydrate, then place it at room temperature to completely After dissolving, add water to 1000ml, shake well, that is.
Embodiment 2
[0029]Calculated by preparing 1000 tablets, the weight of raw and auxiliary materials consists of: boningmycin hydrochloride 1.5g, glucose 270.5g as filler, sodium stearyl fumarate 4g as lubricant, methyl cellulose as binder Su 4g, low-substituted hydroxypropyl cellulose 20g as a disintegrant. Mix the raw materials and auxiliary materials of the above-mentioned prescription amount evenly, and directly compress into tablets to obtain the bonomycin hydrochloride soluble tablet of the present invention, each tablet contains 1.5 mg of bonomycin hydrochloride, and the theoretical tablet weighs 300 mg.
[0030] Preparation of special solvent for soluble tablets (dispersible tablets): Take 20g of sodium alginate, 10g of methylparaben, add appropriate amount of water (about 300ml), stir well to disperse evenly, place at room temperature to dissolve completely, add water to 1000ml, shake Uniform, that is.
Embodiment 3
[0032] Calculated by preparing 1000 tablets, the weight of raw and auxiliary materials consists of: boningmycin hydrochloride 150g, lactose 45g as filler, boric acid 15g as lubricant, maltodextrin 30g as binder, Sodium carboxymethyl starch 60g. Mix the raw materials and auxiliary materials of the above-mentioned prescription amount evenly, and directly compress into tablets to obtain the bonomycin hydrochloride soluble tablet of the present invention, each tablet contains 150 mg of bonomycin hydrochloride, and the theoretical tablet weighs 300 mg.
[0033] Preparation of special solvent for soluble tablets (dispersible tablets): Take 50g of sodium carboxymethylcellulose, 50g of benzyl alcohol, add an appropriate amount of water (about 300ml), stir well to disperse evenly, place at room temperature to dissolve completely, add water to 1000ml, shake Uniform, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com