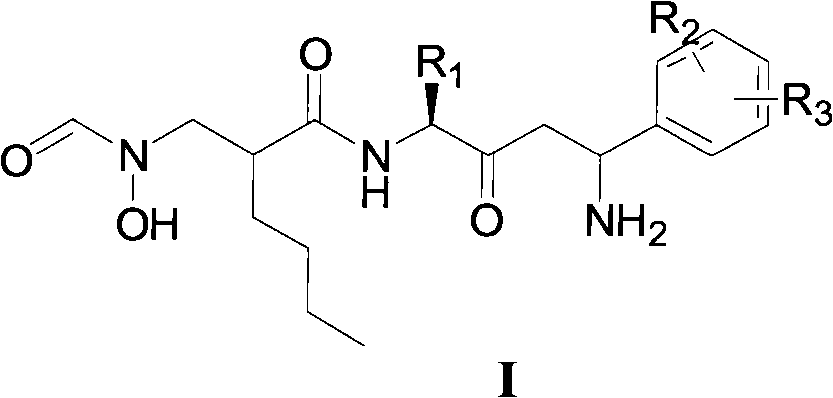
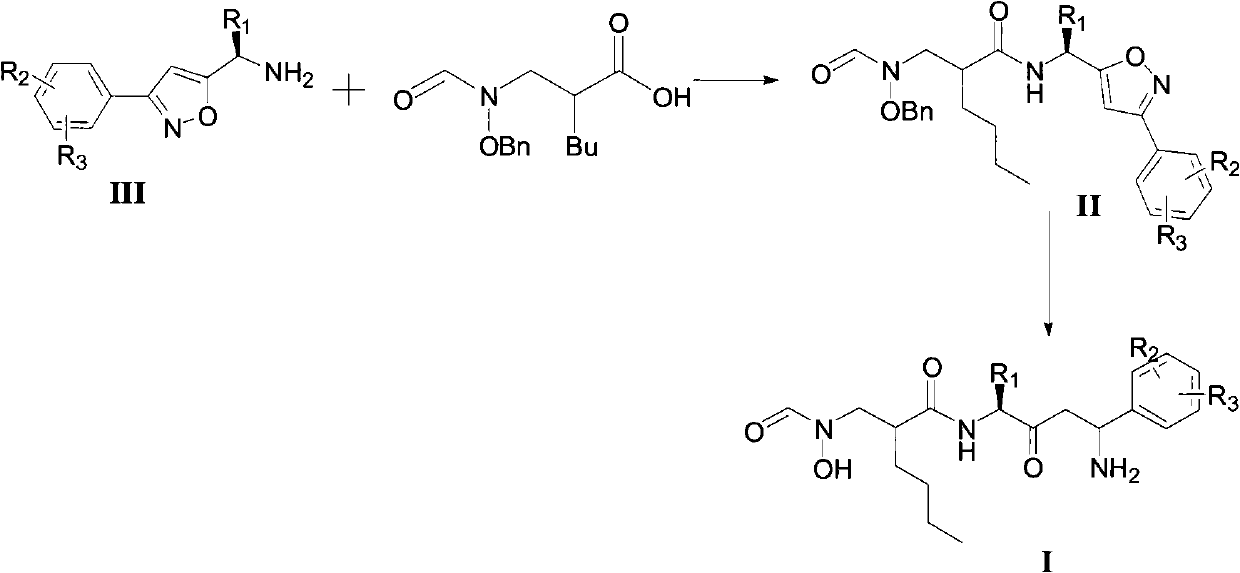
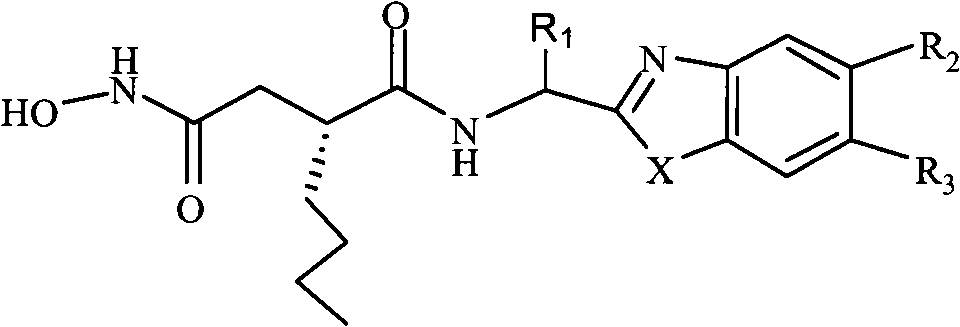
N-formyl hydroxylamine compounds, preparation method, and use thereof
A technology of compounds and mixtures, applied in the field of medicine, can solve the problems of high polarity and easy interconversion of hydroxime groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of 2-[(N-benzyloxy-N-formylamino)methyl]hexanoic acid
[0082] Preparation of acetone oxime: Add 20 mL of water and 6.95 g (0.1 mol) of hydroxylamine hydrochloride to a 100 mL round bottom flask, stir to dissolve the solid, drop in 6.38 g (0.11 mol) of acetone, react at room temperature for 1.5 hours, cool in an ice-water bath, and batch 4.0 g (0.1 mol) of sodium hydroxide was added one at a time, stirred for 0.5 hours, extracted with ether (30 mL×3), dried, and distilled off ether to obtain 6.79 g of white solid, yield 93%, m.p.61-62°C.
[0083] Preparation of oxybenzylacetone oxime: add 8.0 g (0.348 mol) of sodium metal into 150 mL of absolute ethanol, and cool down after all the sodium metal is dissolved. The solution obtained by dissolving 25.4g (0.348mol) of acetone oxime in 100mL of absolute ethanol was added dropwise to sodium ethoxide, and stirred for 1 hour. Then 44.1 g (0.348 mol) benzyl chloride was slowly added dropwise to the reacti...
Embodiment 2
[0092] Example 2 Preparation of 3-phenyl-5-aminomethylisoxazole
[0093] This example is the raw material used to prepare the compound of the present invention: 3-phenyl-5-aminomethylisoxazole, which is one of the compounds represented by formula III.
[0094] The chemical reaction is as follows:
[0095]
[0096] Preparation of benzaldoxime: Add 150mL of water, 50mL of methanol and 10.6g (0.10mol) of benzaldehyde into a 500mL Erlenmeyer flask, stir well and add 8.34g (0.12mol) of NH 2 After OH·HCl was completely dissolved, 6.36 g (0.06 mol) of sodium carbonate was added slowly, stirred at room temperature for 4 h, then 200 mL of water was added, and dichloromethane (60 mL×4) was extracted. The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 11.2 g of a white solid. Yield 97%, m.p. 128-130°C. 1 H NMR (300MHz, CDCl 3 , δppm): 7.40-7.50 (m, 3H, Ar-H), 7.58-7.65 (m, 2H, Ar-H), 8.24 (s, 1H, OH).
[009...
Embodiment 3
[0101] Example 3 Preparation of N-[4-amino-2-carbonyl-(4-phenyl)butyl]-2-[(N-hydroxy-N-formylamino)methyl]hexanamide
[0102]
[0103] To a 50 mL round bottom flask was added 1.0 g (5.72 mmol) CDMT and 1.45 g (5.20 mmol) 2-[(N-benzyloxy-N-formylamino)methyl]hexanoic acid dissolved in dry dichloromethane (30 mL ), add dropwise 683mg (6.76mmol) N-methylmorpholine under ice-salt bath, after reacting for 4 hours, add 2.26g (5.20mmol) 3-phenyl-5-aminomethylisoxazole, After reacting for 2 hours, react overnight at room temperature. Remove the precipitate by filtration, wash the precipitate with a small amount of dichloromethane, combine the washing liquid and the filtrate, and successively wash with 0.5mol / L hydrochloric acid, saturated NaHCO 3 , saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was separated on a silica gel column (dichloromethane:methanol=20:1) to obtain 1.56 g of a white solid, with a yield of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com