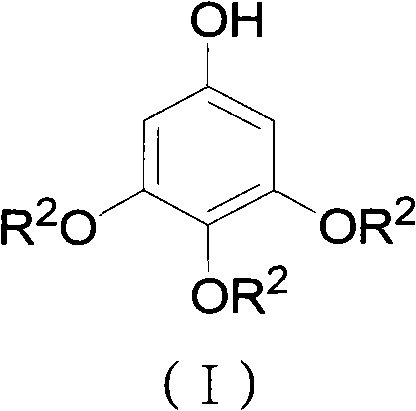
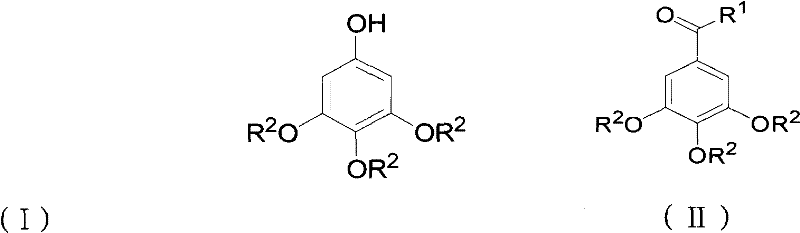
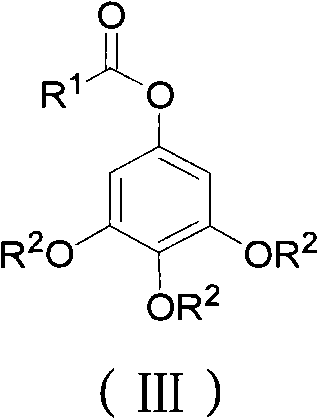
Preparation method for 3,4,5-trialkoxy phenol
A technology of methyl and ethyl, which is applied in the field of preparation 3, can solve the problems of unsuitable industrial production and low yield of phenol, and achieve the effects of avoiding reaction by-products, simplifying process steps, and achieving significant industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Measure 35ml of dimethyl carbonate and 5ml of methanol into a 100ml reaction bottle, add 0.5g of 98% concentrated sulfuric acid and 2.1g of raw material 3,4,5-triethoxyphenyl methyl ketone successively, stir and dissolve at room temperature Then add 4ml of 30% hydrogen peroxide dropwise. The reaction mixture was stirred at room temperature for 20 hours, and then the reaction was terminated after TLC checked that the raw materials were completely reacted.
[0035] In addition, about 2.0 g of sodium sulfite was weighed and dissolved in 10 ml of water, and then slowly added to the above reaction solution, using sodium sulfite to neutralize excess hydrogen peroxide. After cooling down slightly, slowly add sodium bicarbonate solution until there is no obvious gas overflow or the pH of the aqueous phase is alkaline, about 0.84g is needed (dissolve in about 5-10ml of water and add). Because also used excessive strong acid catalysis in the reaction, adopt sodium bicarbonate to...
Embodiment 2
[0037] Measure 44ml of dimethyl carbonate and 6ml of ethanol into a 100ml reaction bottle, add 1g of 96% phosphoric acid and 1.2g of raw material 3,4,5-trimethoxybenzaldehyde successively, stir and dissolve at room temperature, then add dropwise 1.2ml of hydrogen peroxide. The reaction mixture was stirred at room temperature for 16 hours, and then the reaction was terminated after TLC checked that the raw materials were completely reacted.
[0038] Another weighed about 1.26g of sodium sulfite was dissolved in 10ml of water and slowly added to the above reaction solution. After cooling down slightly, slowly add sodium bicarbonate solution until there is no obvious gas overflow or the pH of the aqueous phase is alkaline, about 1.6g is needed. After fully stirring, 10ml of chloroform was added, and the mixture was left to stand to separate layers after continuing to stir. The organic phase was separated, and the aqueous phase was extracted once more with 15 ml of chloroform. ...
Embodiment 3
[0040] Measure 50ml of dimethyl carbonate and 5ml of methanol into a 100ml reaction bottle, add 1g of 96% sulfuric acid and 2.4g of raw material 3,4,5-triethoxybenzaldehyde successively, stir and dissolve at room temperature, then add 4.8ml dropwise of hydrogen peroxide. The reaction mixture was stirred at room temperature for 12 hours, and then the reaction was terminated after TLC checked that the raw materials were completely reacted.
[0041] Another weighed about 2.3g of sodium sulfite was dissolved in 10ml of water and slowly added to the above reaction solution. After cooling down slightly, slowly add sodium bicarbonate solution until there is no obvious gas overflow or the pH of the aqueous phase is alkaline, about 1.6g is needed. After fully stirring, add 10ml of dichloromethane, continue to stir and then let stand to separate layers. The organic phase was separated, and the aqueous phase was extracted once more with 15 ml of dichloromethane. The organic phases wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com