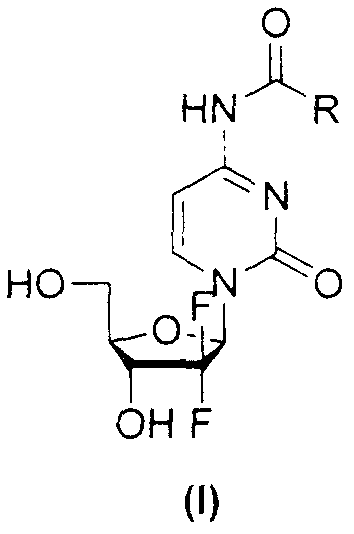
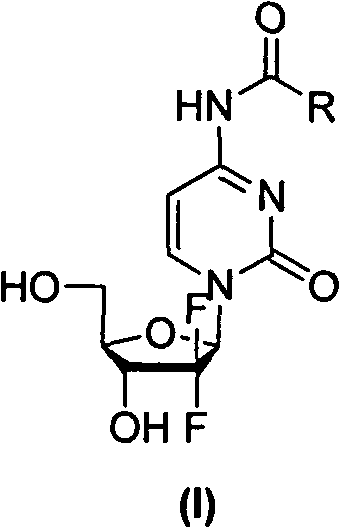
Gemcitabine amide derivates, and preparation method and application thereof
A technology of amides and derivatives is applied in the field of preparing antitumor drugs and gemcitabine amide derivatives, which can solve the problems of poor oral bioavailability and intestinal damage of gemcitabine, and achieve the effect of good antitumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
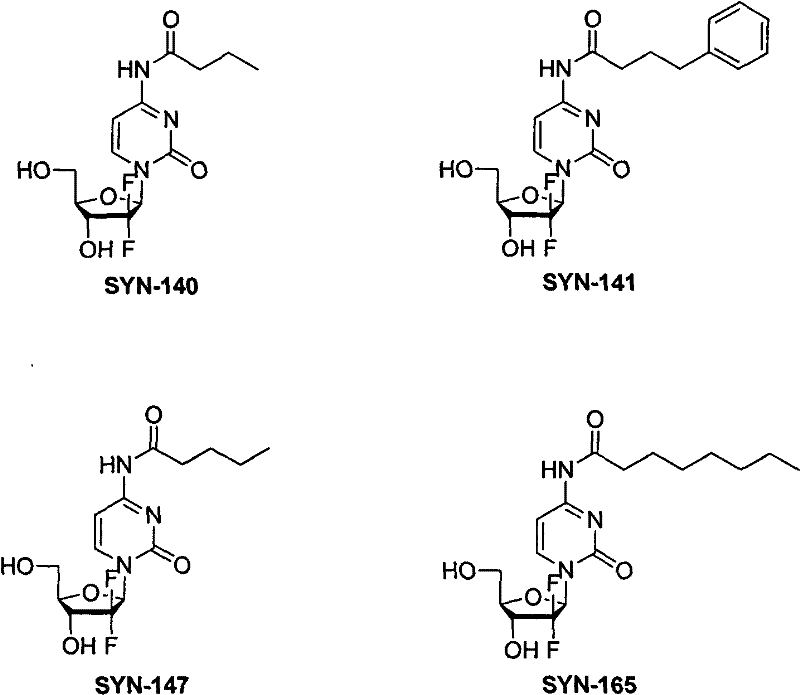
[0028] Example 1: N 4 - Synthesis of n-butyryl gemcitabine (SYN-140)
[0029] 0.44 g (1.67 mmol) of gemcitabine, 5 mL of anhydrous pyridine, and 1.1 mL of triethylchlorosilane were sequentially added into a 50 mL round bottom flask, and stirred at room temperature for 1.5 hours. Obtain solution A; at the same time, dissolve 0.16g (1.81mmol) of n-butyric acid in 4mL of acetonitrile, add 0.33g (2.03mmol) of carbonyldiimidazole, stir at room temperature for 0.5 hours, drop this solution into solution A, and keep warm at 60°C Stir overnight, remove the solvent from the reaction solution under reduced pressure, dissolve the residue in 5 mL of methanol, add 1 mL of trifluoroacetic acid dropwise, stir for 0.5 hours, pour 50 mL of ethyl acetate, collect the precipitated solid, wash the solution with saturated brine 15 mL×2, Wash once with water, dry the organic phase with anhydrous sodium sulfate, reclaim the solvent, combine the residue and the previously precipitated solid to obtai...
Embodiment 2
[0031] Example 2: N 4 -Synthesis of phenylbutyryl gemcitabine (SYN-141)
[0032] Prepare with reference to the method of Example 1.
[0033] MP: 111°C, 1 H NMR (DMSO-d6) δ: 11.0 (1H, s), 8.22 (1H, d, J = 7.8Hz), 7.27 (3H, m), 7.18 (3H, m), 6.29 (1H, d, J = 6.6Hz), 6.16(1H, t, J=7.2Hz), 5.28(1H, brs), 4.18(1H, m), 3.88(1H, m), 3.80(1H, m), 3.65(1H, m) , 2.58(2H, t, J=7.2Hz), 2.45(2H, m), 1.87(3H, t, J=7.2Hz), ESIMS m / z(relintensity): 410(M+H + , 100).
Embodiment 3
[0034] Example 3: N 4 -Synthesis of n-valeryl gemcitabine (SYN-147)
[0035] Prepare with reference to the method of Example 1.
[0036]MP: 184°C, 1 H NMR (DMSO-d6) δ: 10.96 (1H, s), 8.22 (1H, d, J = 7.8Hz), 7.27 (1H, d, J = 7.8Hz), 6.29 (1H, brs), 6.16 (1H , t, J=7.2Hz), 5.28(1H, brs), 4.18(1H, m), 3.88(1H, m), 3.79(1H, m), 3.63(1H, m), 2.40(2H, t, J=7.2Hz), 1.52(2H, m), 1.28(2H, m), 0.86(3H, t, J=7.2Hz), ESIMSm / z(relintensity): 348(M+H + , 100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com