Dry heat treatment method for human coagulation factor VIII preparation and dry heat treatment stabilizer
A human blood coagulation factor, dry heat treatment technology, applied in the field of medicine, can solve the problems of human blood coagulation factor VIII titer loss, increase production cost, titer loss, etc., achieve strong operability and controllability, reduce activity loss, The effect of improving the activity recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
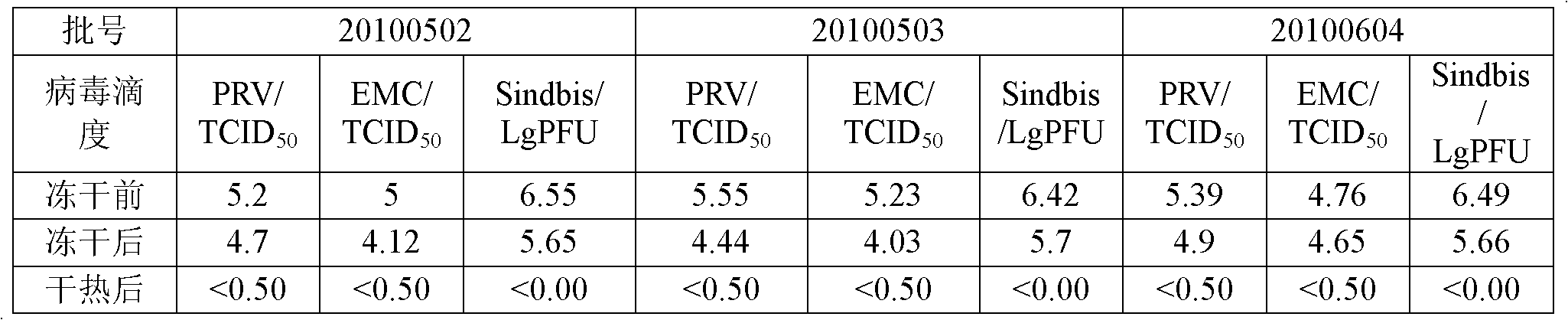
[0023] Example 1: Verification of virus inactivation after adding stabilizer
[0024] (1) Preparation of human coagulation factor VIII
[0025] Centrifuge the plasma at 0-4°C to obtain cryoprecipitate, dissolve the cryoprecipitate, undergo acid precipitation and clarification and filtration, add S / D solution to the final concentration of tween80 1%, TNBP 0.3%, incubate at 24-26°C for 6 hours, and then pass through the column Chromatography obtains a protein solution containing human coagulation factor VIII, and the resulting eluate is dialyzed with a solution containing 0.01-0.1M NaCl, 0.01M trisodium citrate, 0.1%-0.349% lysine hydrochloride, and 0.1%-10% glycine The solution was subjected to ultrafiltration, desalting and dialysis to obtain a concentrated solution of human coagulation factor VIII.
[0026] (2) Add stabilizer to prepare human blood coagulation factor VIII freeze-dried agent
[0027] Add 20% human serum albumin to the concentrated solution to a concentration...
Embodiment 2
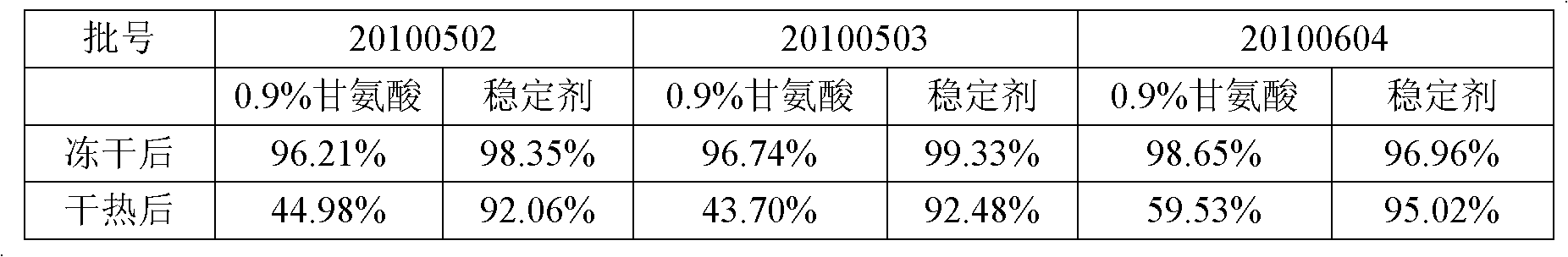
[0033] Example 2: The influence of human blood coagulation factor VIII stabilizer on preparations in dry heat inactivation at 80°C for 72 hours
[0034] When preparing the human blood coagulation factor VIII concentrate, the dialysate was concentrated and dialyzed with 0.9% glycine and the prepared stabilizer as the dialysate, and the protection of the stabilizer to the human blood coagulation factor VIII preparation in freeze-drying and dry heat inactivation was observed The recovery rate of potency after lyophilization and dry heat inactivation is as follows:
[0035] Table 2 Human blood coagulation factor VIII stabilizer protection experiment result
[0036]
[0037] It can be seen from Table 2 that when no stabilizer is added and only 0.9% glycine is included as an excipient, the titer loss is as high as 40%-50%, and after adding a stabilizer, the potency loss rate is higher than 90%.
Embodiment 3
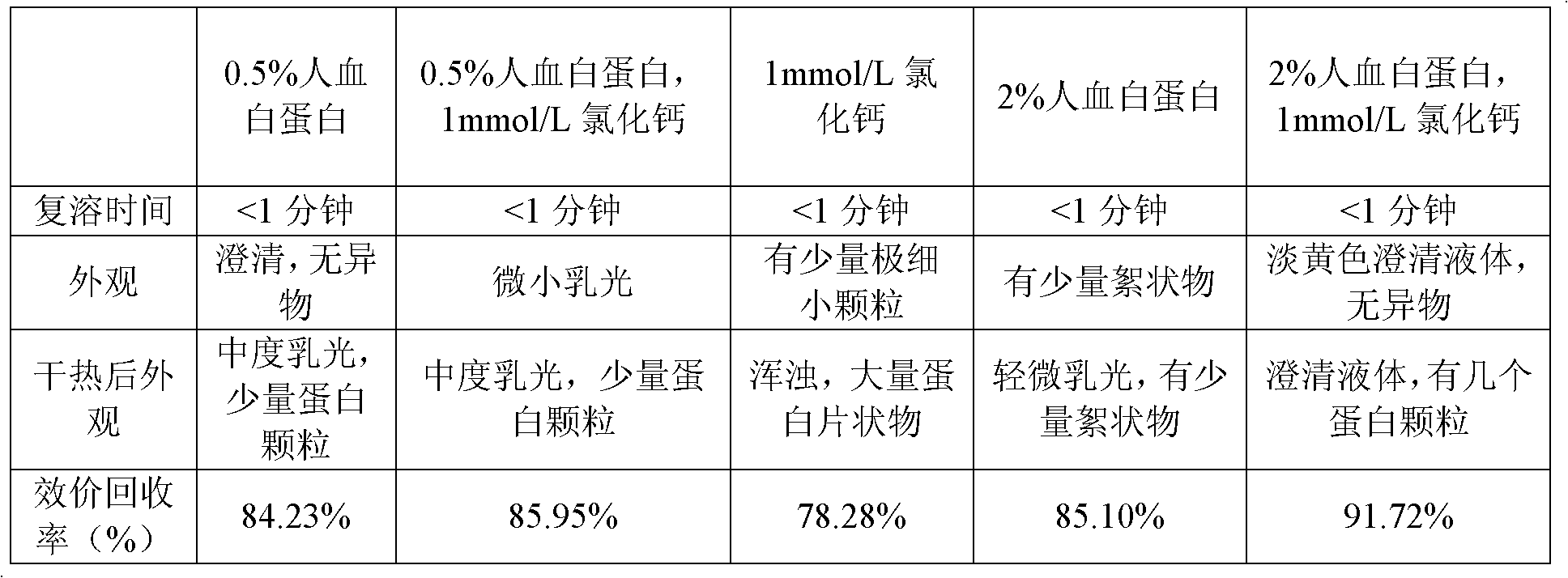
[0038] Embodiment 3: human serum albumin and Ca in the dialysate 2+ Protection against human coagulation factor VIII.
[0039] 1. Prepare human coagulation factor VIII according to conventional methods, except CaCl 2 In addition to human albumin, a stabilizer is added according to the standard to prepare a concentrated solution of human blood coagulation factor VIII preparation.
[0040] 2. Observe the clarity of the sample after reconstitution and the activity recovery rate of human coagulation factor VIII
[0041] The concentrated solution prepared by the above method was freeze-dried with the human blood coagulation factor VIII added with the stabilizer as shown in the table below, tested at 80°C for 72 hours, observed the clarity of the solution, and tested the potency to observe the effect of the protective agent on virus inactivation. Effects of human coagulation factor VIII. The results are shown in Table 3.
[0042] Table 3: Research results of the final solution o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com