Inhalation serrapeptase preparation
A technology of serrapeptase and inhalation drug delivery, which is applied in the field of medicine and can solve problems such as low bioavailability, slow onset of action, and unsatisfactory therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Aerosol for inhalation:
[0009] Recipe and process: Take the main medicine into micropowder, the particle size D90 is less than 10 microns, weigh 0.5g of the micronized powder, put it into aluminum cans, lock the metering valve, and fill it with 12g of tetrafluoroethane. Shake well before use, each press 100μl, containing 5mg of main medicine.
Embodiment 2
[0011] Powder mist for inhalation:
[0012] Prescription and process: take the main drug into micronized powder, the particle size D90 is less than 10 microns, weigh 5g of the micronized powder, 150g of lactose for aseptic inhalation, mix well in a sterile environment, and fill in the No. 3 capsule shell, and the preparation is complete. 1000 tablets. Put 1 capsule into a special inhalation device before use and set aside. Each capsule shell contains 5mg of the main drug.
Embodiment 3
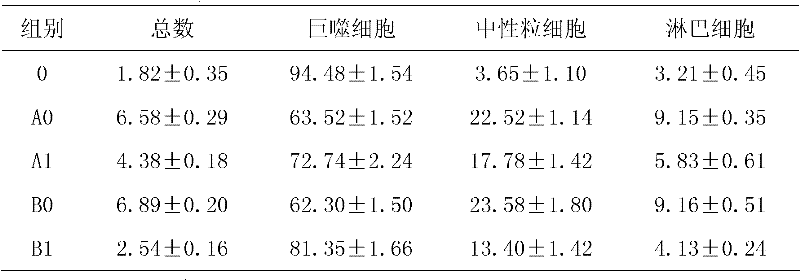
[0013] Example 3: Influence on airway inflammation
[0014] Experimental method: 40 rats were randomly divided into two groups, 20 rats in each group. They are the oral administration group (group A) and the inhalation administration group (group B). Group A is randomly divided into: blank control group (A0) and oral serrapeptase group (A1), group B is randomly divided into They are: blank control group (B0) and oral serrapeptase group (B1). In each group, 1 mg / kg lipopolysaccharide was injected into the tube on the 1st and 20th day of the experiment, and the rats were put into a 80cm×60cm×58cm plexiglass box for passive smoking on the 2nd to 19th and 21st to 40th days, twice a day (interval 4h ), each lasting 1h (10 cigarettes). Each treatment group was given corresponding treatment drugs on the 2nd to 19th and 21st to 40th days. Inhalation group: Therapeutic drug + 5ml physiological saline, inhaled at 5L / min in aerosol, (placed in a 30cm×30cm×20cm glass box), 3 times / d, 25mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com