Real-time fluorescence quantitative PCR(Polymerase Chain Reaction) detection primers and kit for thalassemia
A real-time fluorescence quantitative, thalassemia technology, applied in the field of medical detection, can solve the problems of affecting the resolution, distorted results, inability to achieve, etc., to achieve high detection sensitivity, accurate and reliable results, and meet the effect of rapid detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The thalassemia gene diagnostic kit described in this embodiment,
[0083] Include primers from Table 2:
[0084] Table 2. Primers used for HBB gene in HRM mutation screening.
[0085]
[0086]
[0087] Fluorescent dye component: LC Green Plus.
[0088] Other dyes such as LC green, SYTO 9, Eva Green or other saturating fluorescent dyes can also be used in the present invention.
[0089] Experimental procedure
[0090] (1) DNA extraction: 2 ml of peripheral venous blood was collected using EDTA anticoagulant tubes. Genomic DNA was extracted using a DNA extraction kit, and diluted to 10-20 ng / μl after quantification.
[0091](2) Primer design: According to the gene mutation characteristics of HBB in southern my country, 6 pairs of primers were designed (Table 1, Figure 7 ), in the design, according to the inventor's rich experience and a large number of experiments, design primers that cover as many known β-thalassaemia mutation sites in the Chinese population...
Embodiment 2
[0111] Genetic diagnosis of non-deletion alpha-thalassemia.
[0112] The common non-deletion α-thalassemia genotypes in China are CS (constant spring), QS (Quong Sze) and WS (Westmead).
[0113] Experimental procedure
[0114] (1) DNA extraction: 2 ml of peripheral venous blood was collected using EDTA anticoagulant tubes. Genomic DNA was extracted using a DNA kit, and diluted to 10-20ng / μl after quantification.
[0115] (2) Primer Design: Primer Forward:
[0116] 5'-CACTGCCTGCTGGTGACC-3' (SEQ ID.NO.13)
[0117] Reverse: 5'-GCAGGAGGAACGGCTACC-3' (SEQ ID.NO.14)
[0118] (3) Use 96-well plates (for Roche480) for PCR amplification, the amplification system is 20 μl: 1 μl (about 10-20ng) of genomic DNA or 10 3 Copy / ml plasmid 1 μl, 5×reaction buffer, dNTP 250 μM, primers 0.2 μM, 1×LC Greens PLUS dye (Idaho Technology), Promega polymerase 1.0 U, add sterilized distilled water to 20 μL. Cycle conditions: 95°C for 3min; 98°C for 10s, 60°C for 10s, 72°C for 20s, 33 cycles.
[0...
Embodiment 3
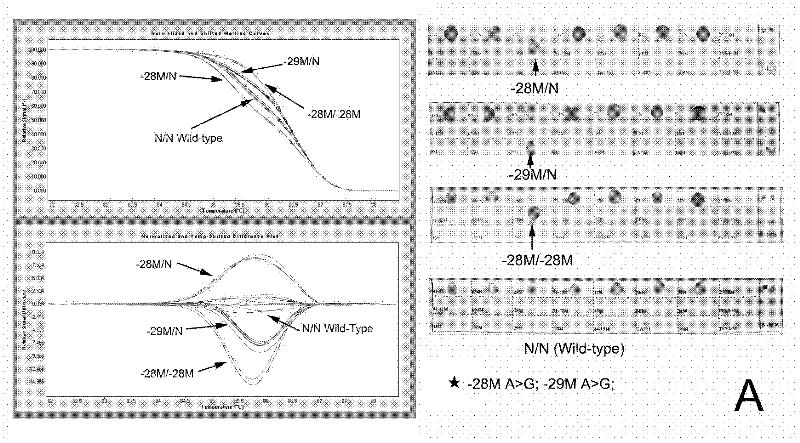
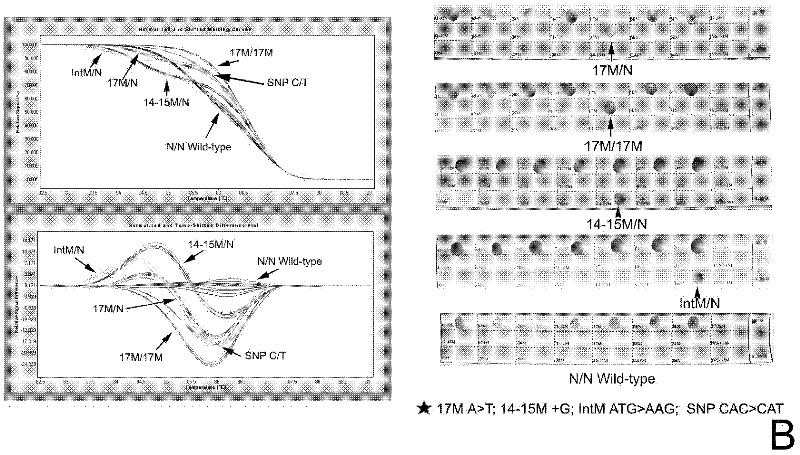
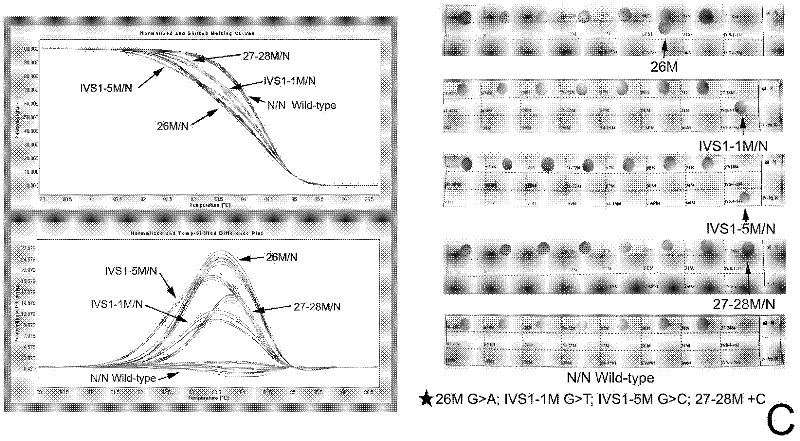
[0125] Multiple samples of thalassemia of different genotypes were collected, and the detection methods of Examples 1 and 2 were used to detect α-thalassemia and β-thalassemia together. The PCR reaction system and HRM analysis were the same as above. At the same time, the samples were verified by DNA sequencing and RDH (Shenzhen Yaneng Reverse Dot Hybridization Kit).
[0126] Table 3.1. β-Thalassaemia The number of specimens detected by HRM analysis, gene sequencing and Shenzhen Yaneng reverse dot blot kit
[0127]
[0128]
[0129] Table 3.2. The number of specimens detected by HRM analysis, gene sequencing and Shenzhen Yaneng reverse dot blot kit for α-thalassemia
[0130]
[0131] From the above results, it can be seen that the thalassemia gene diagnosis kit, the primers and the test kit for thalassemia gene diagnosis of the present invention have good detection specificity and high sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com