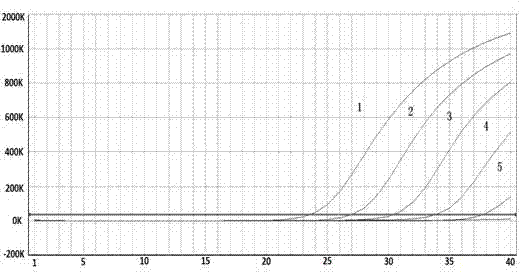
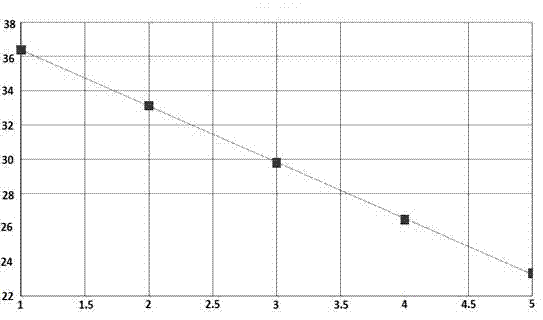
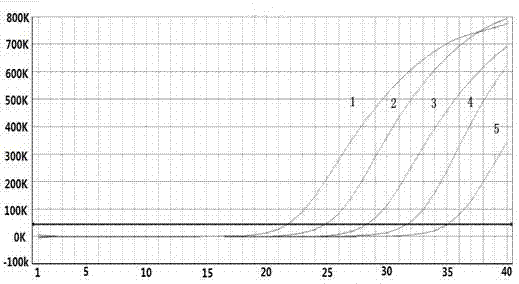
Quadruple fluorescent quantitative polymerase chain reaction (PCR) detection kit for diarrheagenic escherichia coli
A detection kit and fluorescent quantitative technology, applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problems of cumbersome operation, long time-consuming, time-consuming and labor-intensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1 Materials and methods
[0046] 1.1 Strains and clinical specimens:
[0047] Diarrhea-causing Escherichia coli O157:H7 and O104:H4 bacteria (the strains were identified by automatic biochemical analyzer and agglutinated by Japanese Shengken and Danish serum) were purchased from the Chinese Center for Disease Control and Prevention. Clinical samples were obtained from fecal specimens of recent patients. After sample collection, they were stored at low temperature and transported to the laboratory in time.
[0048] 1.2 Primers and probes
[0049] The gene sequences of diarrhea-causing Escherichia coli O157:H7 and O104:H4 from around the world were downloaded from the NCBI gene bank in the United States. The homology comparison was carried out, and specific primers and Taqman probes were designed in the conserved gene region of the corresponding genome. The sequences are as follows:
[0050] O157 upstream primer-F: 5'-TGGCATGACGTTATAGGCTACAAT-3'
[0051] O157 Downstre...
Embodiment 2
[0079] Example 2 Detection of clinical samples
[0080]Using the Diarrhea Syndrome Questionnaire of the "Eleventh Five-Year" Major Project-Infectious Disease Pathogen Monitoring Technology Platform, 800 outpatient diarrhea specimens (daily Defecation 3 or more times, and the stool is loose stool, watery stool, mucus stool or pus and blood stool and other characteristics have changed) for testing. Bacterial DNA is directly extracted from the collected diarrhea clinical samples, and the fluorescent PCR method of the present invention is used to detect the target pathogenic bacteria; at the same time, the conventional culture method is used to conduct parallel experiments. Results The fluorescent PCR method detected one part of O157:H7 and two parts of O104:H4. The fluorescent PCR method of the present invention has a higher positive rate than the conventional culture method and is easy to operate. The verification of the fluorescence PCR method of the present invention for clin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com