Oligopeptide derivative capable of combination of basic fibroblast growth factor, and application thereof
A technology of fibroblasts and growth factors, which can be applied in the fields of peptides, drug combinations, anti-tumor drugs, etc., and can solve problems such as reports of peptide derivatives antagonists that have not yet been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] peptide synthesis
[0028] By the solid-phase peptide synthesis method, a 413A automatic peptide synthesizer (purchased from Perkin Elmer) was used to synthesize the peptide shown in the following sequence: N'-Pro-Ile-Leu-Gln-Ala-Gly-Leu-Gly- Gly-Gly-Ser-NH2-C', wherein the amino acid residues are all L-type amino acids. The specific process of synthesis is as follows: Rink Amide-AM Resin resin is used as the carrier; Fmoc is used as the protecting group of the amino acid α-amino group; DMF is used as the reaction solvent; the activator is HBTu: 0.5M (DMF) and NMM (N-methylmorpholine ), 2M (DMF); DMF containing 20% piperidine was used as the deprotecting agent; the lysate was 95% TFA and 5% (H2O+EDT+Tis). Peptides were precipitated with anhydrous ether. Precipitation in water / tert-butanol (1:1) and lyophilization afforded the crude peptide. The crude peptide was purified by reverse phase HPLC in 30 minutes with a gradient of 37-42% acetonitrile / 0.9% TFA. It was th...
Embodiment 2
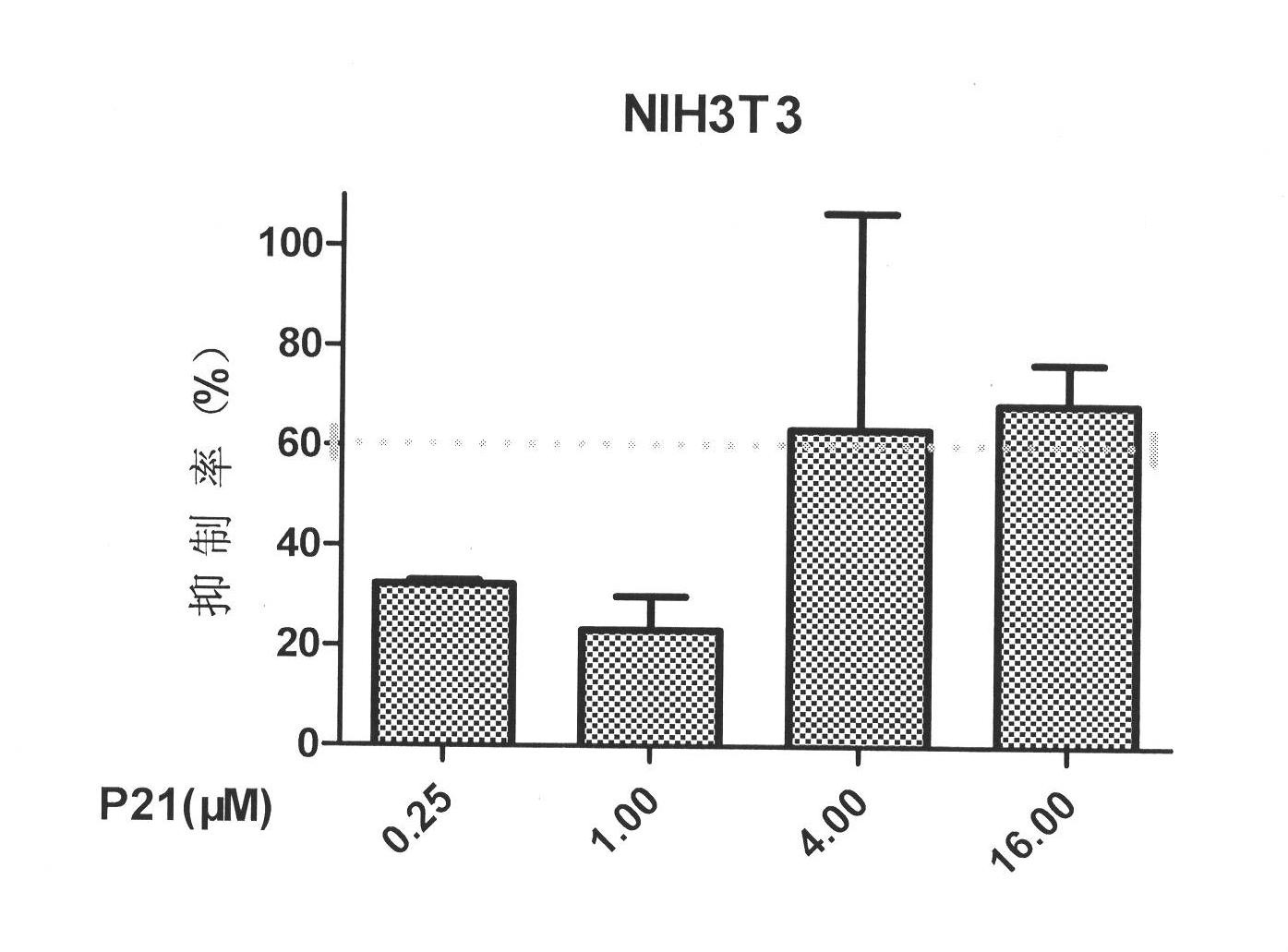
[0030] Inhibitory activity of P21 on the proliferation of NIH3T3 cells induced by bFGF
[0031] Select NIH3T3 cells expressing more bFGF receptors, inoculate 6000 cells / well in 96-well culture plate, 100 μl per well, 10% FBS (imported) DMEM low-glucose medium; after overnight, replace with 0.1% FBS The DMEM low-glucose medium was cultured for 24 hours; the mixture of bFGF and P21 was added, the final concentration of bFGF was 20 ng / ml, and the final concentrations of peptides were 0.25, 1.00, 4.00, and 16.00 μM, respectively. After 48 hours, the OD value at 490nm was measured by MTT method, and the inhibition rate was calculated. The test includes: blank group, no bFGF and peptide; negative group, only bFGF (20ng / mL) and no peptide; drug-dosed test group; blank group and negative group are added with the same volume of PBS as the drug-dosed test group . The calculation formula of inhibition rate=[OD (negative group)-OD (test group)] / [OD (negative group)-OD (blank group)]*100...
Embodiment 3
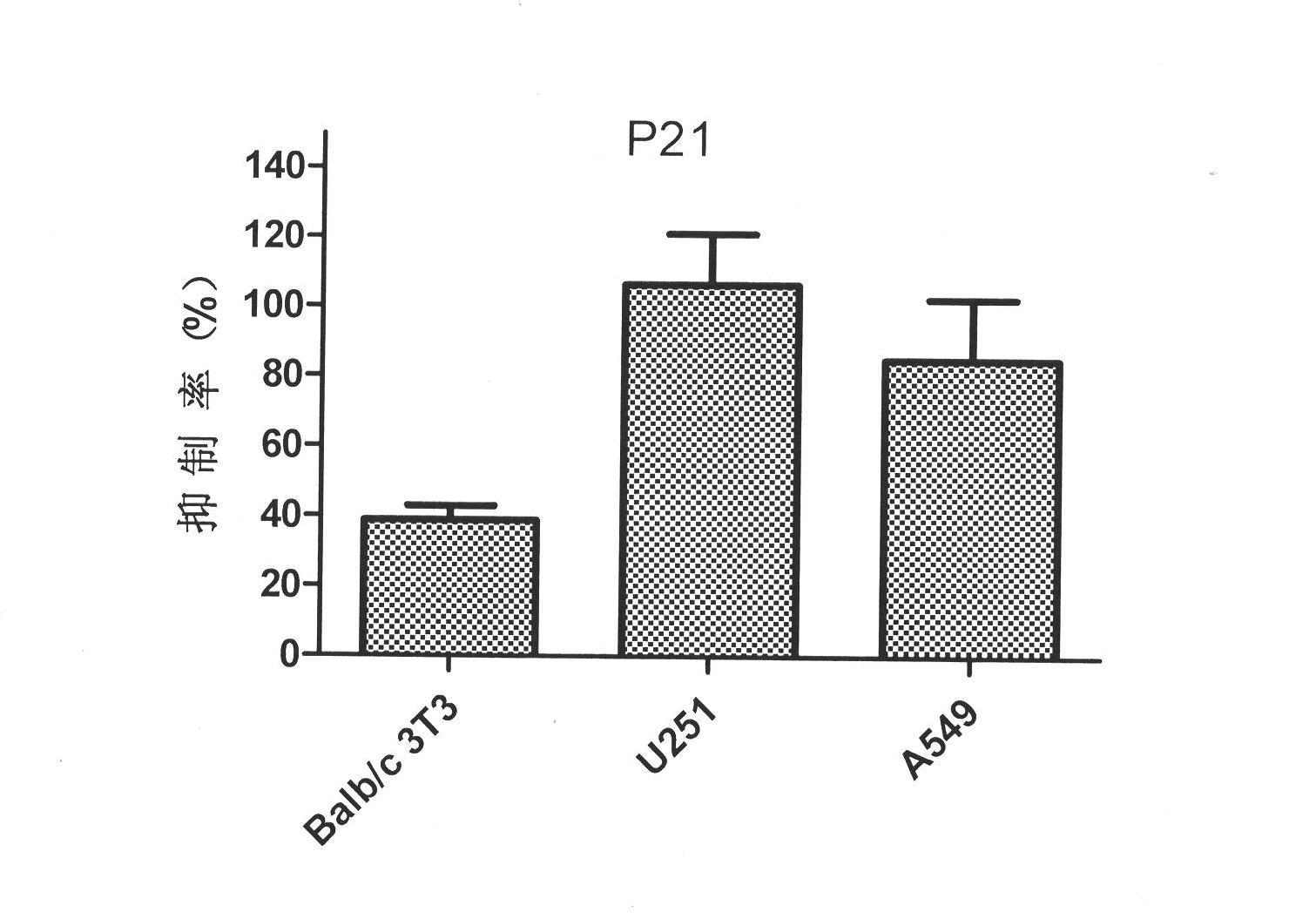
[0033] Inhibitory activity of P21 on bFGF-induced cell proliferation
[0034] Select Balb / c 3T3, lung cancer (A549) cells, and glioma (U251) cells expressing more bFGF receptors, and inoculate 6000 cells / well in 96-well culture plates, 100 μl per well, medium Use DMEM high-glucose medium with 10% FBS; after overnight, replace with 0.1% FBS medium for 24 hours; add the mixture of bFGF and P21, the final concentration of bFGF is 20ng / ml, and the final concentration of peptide is 4.00μM . After 48 hours, the OD value at 490nm was measured by MTT method, and the inhibition rate was calculated. The test includes: blank group, no bFGF and peptide; negative group, only bFGF (20ng / mL) and no peptide; drug-dosed test group; blank group and negative group are added with the same volume of PBS as the drug-dosed test group . The calculation formula of inhibition rate=[OD (negative group)-OD (test group)] / [OD (negative group)-OD (blank group)]*100%. For inhibitory activity data on cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com