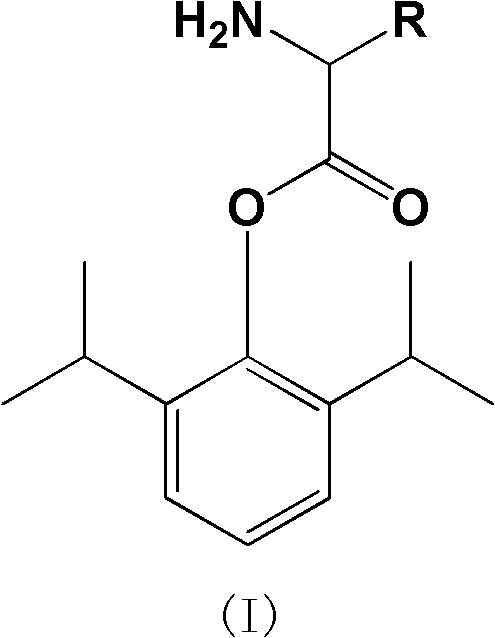
Water-soluble amino acid ester derivative of propofol and application thereof
A technology of amino acid esters and derivatives of propofol, which can be used in drug combination, cyanide reaction preparation, organic compound preparation, etc., and can solve problems such as unfavorable drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0062] The present invention can be further described by the following examples, however, the scope of the present invention is not limited to the following examples.
[0063] Those skilled in the art can understand that various changes and modifications can be made in the present invention without departing from the spirit and scope of the present invention. The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible.
[0064] The protected amino acids and condensation reagents used in the examples are products of Shanghai Jier Biochemical Company; conventional solvents and reagents are commercial products.
Embodiment 1
[0066] Synthesis of 2-L-lysine-1,3-diisopropyl-phenyl ester dihydrochloride (compound 1)
[0067]
[0068] Compound 1
[0069] Under water bath conditions, N α -tert-butoxycarbonyl-N ε -tert-butoxycarbonyl-lysine (2.2mmol, 0.76g), N, N'-dicyclohexylcarbodiimide (4.4mmol, 0.91g), 4-dimethylaminopyridine (0.22mmol, 0.03g ), stirred and dissolved with anhydrous dichloromethane (30ml). Then propofol (2.6mmol, 0.46g) was added and stirred overnight at room temperature. The reaction was monitored by HPLC until the raw material was completely reacted. The insoluble solid was removed by filtration, and the filtrate was evaporated to dryness; ether was added to the residue, and insoluble impurities in the solution were removed by filtration. The filtrate was concentrated and quickly passed through the column with diethyl ether as the developing solvent to collect product fractions. Concentrate under reduced pressure to obtain the product. The obtained product was directly a...
Embodiment 2
[0073] Synthesis of 2-L-arginine-1,3-diisopropyl-phenyl ester dihydrochloride (compound 2)
[0074]
[0075] Compound 2
[0076] Synthetic method with reference to embodiment 1, with N α - tert-butoxycarbonyl-arginine hydrochloride replaces N α -tert-butoxycarbonyl-N ε - tert-butoxycarbonyl-lysine. Compound 2 was obtained with a yield of 62.1%.
[0077] ESI-MS m / z: 335 (M+1 + ).
[0078] 1 H NMR (D 2 O)δ0.98(d,12H,-C H 3 ), 1.6-1.7 (m, 2H, CH 2 -C H 2 -CH 2 - δ NH-), 1.8-2.0 (m, 1H, C H ), 2.2-2.2 (m, 1H, C H ), 2.99(t, 2H, C H 2 -CH 2 -CH 2 - δ NH-), 3.2-3.3 (m, 2H, CH 2 -C H 2 - δ NH-), 4.41 (dd, 1H, C H - α NH 2 ), 7.1-7.3 (m, 3H, Ar H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com