Method for preparing anode material lithium vanadium phosphate by adopting quenching
A technology of lithium vanadium fluorophosphate and cathode material, applied in battery electrodes, electrical components, circuits, etc., can solve the difficulty of diffusion and migration of lithium ions and electrons, affect the high-rate charge and discharge performance of materials, and limit the substantiality of lithium vanadium fluorophosphate. application and other issues, to achieve the effect of shortening the diffusion distance, excellent high-rate charge-discharge performance, and improved electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
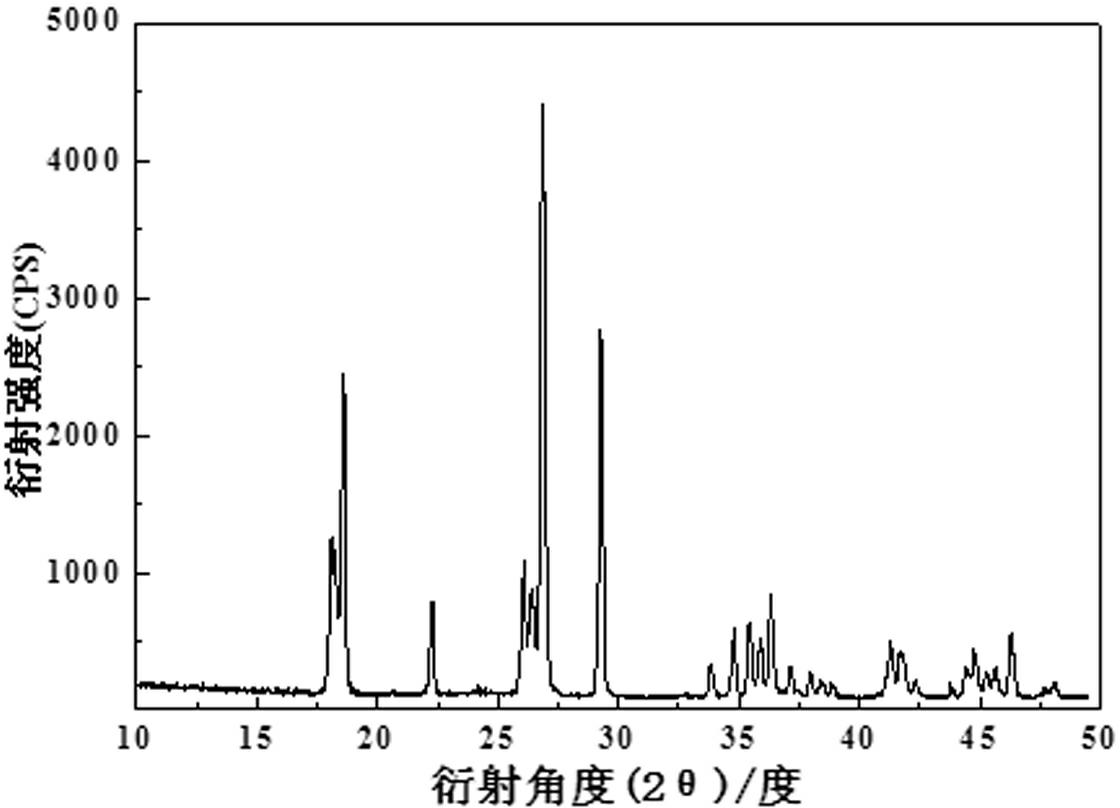
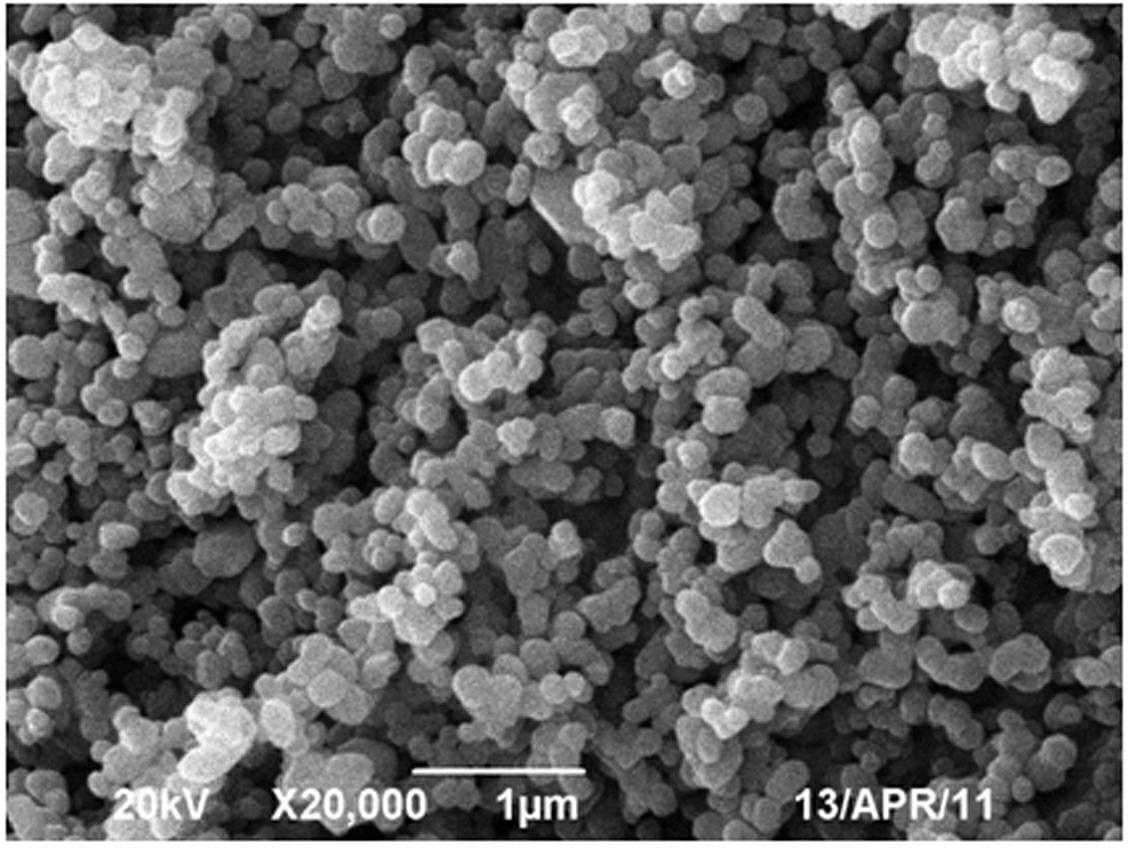
[0017] Mix 0.1mol of vanadium pentoxide, 0.2mol of ammonium dihydrogen phosphate, 0.105mol of lithium carbonate, 0.2mol of hydrofluoric acid and 0.2mol of citric acid through high-energy ball milling, then dry at 80°C, and then put them into tubes In a type furnace, under an argon atmosphere, the temperature was calcined at 500°C for 0.5 hours, and then the high-temperature charge was quickly placed in liquid nitrogen at -193°C, dry ice at -80°C, and water at 0°C. Diffraction analysis shows triclinic crystal system, which is LiVPO 4 F's
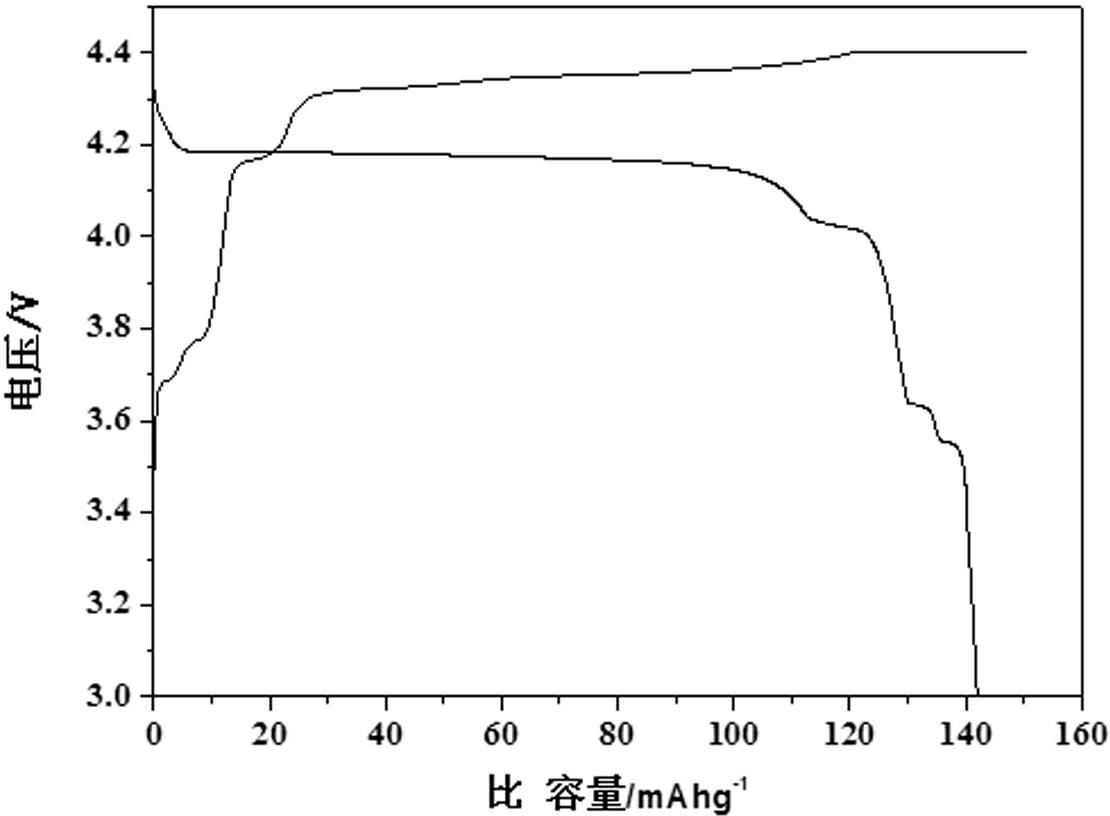
[0018] structure. The particle size obtained by SEM is about 50nm. The obtained product was assembled into a button battery and charged and discharged at a rate of 10C. The first discharge capacity under different conditions is shown in Table 1:
[0019] Table 1
[0020]
Embodiment 2
[0022] Mix 0.1 mol of vanadium dioxide, 0.1 mol of lithium fluoride, 0.1 mol of triammonium phosphate and 1 mol of oxalic acid under high-energy ball milling conditions, dry at 60°C, and then put them into a tube furnace, under hydrogen atmosphere, The temperature was calcined at a constant temperature of 700°C for 0.5h, and then the high-temperature charge was quickly placed in 40°C water to quench for 1min, 20min and 1h. The obtained material was analyzed by X-ray diffraction as a triclinic crystal system, namely LiVPO 4 The structure of F. The particle size obtained by SEM is about 80nm. The resulting product was assembled into a button battery and charged and discharged at a rate of 10C. The first discharge capacity under different conditions is shown in Table 2:
[0023] Table 2
[0024]
Embodiment 3
[0026] Mix 0.1mol of ammonium metavanadate, 0.051 of lithium oxide, 0.1mol of diammonium hydrogen phosphate, 0.1mol of hydrofluoric acid and 0.2mol of sucrose through high-energy ball milling, dry at 120°C, and then put them into a tube furnace. Under the atmosphere, it was calcined at a constant temperature of 800°C for 5 hours, and then the high-temperature charge was quickly placed in dry ice at -87°C for 1 minute, and the obtained material was analyzed by X-ray diffraction as a triclinic crystal system, namely LiVPO 4 The structure of F. The particle size can be found to be about 100nm by SEM. The obtained product was assembled into a button battery and charged and discharged at a rate of 10C, and the specific capacity of the first discharge was 138mAh·g -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com