Method for preparing tolvaptan intermediate
A technology of inorganic alkali and oxo substitution, applied in the direction of organic chemistry, can solve the problems of unreacted raw materials, difficult recrystallization and removal, affecting purification and yield, etc., to achieve increased yield, suitable for large-scale industrial production, and products The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
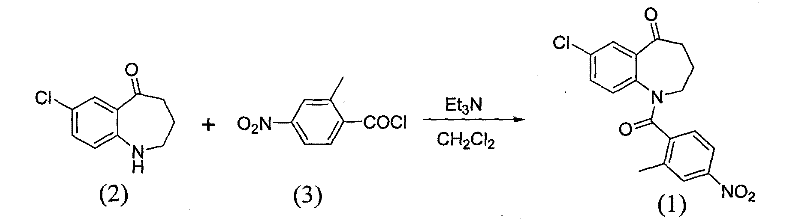
[0015] Add 12.04g (0.0664mol) of 4-nitro-2-methylbenzoic acid into a 100ml single-necked flask, add 9.49g (1.2eq) of thionyl chloride and 12ml of toluene, then add 3 drops of DMF, and reflux for 4 After 1 hour, the solvent and excess thionyl chloride were distilled off under reduced pressure to obtain 13.3 g of a brownish-yellow oil, which was condensed to a solid; dissolved in 5 ml of acetonitrile for later use.
[0016] Add 10g (0.051mol) of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine into a 100ml three-necked flask, dissolve it with 50ml of acetonitrile, and then Add 2.04g (1eq) NaOH, stir, add dropwise 13.3g of 4-nitro-2-methylbenzoyl chloride in 5ml acetonitrile solution, react at room temperature, as the reaction progresses, the reaction solution gradually changes from dark green to light green At the same time, a large amount of white solids were precipitated. After 60 minutes, the dropwise addition was completed, and the reaction was stopped. The reaction soluti...
Embodiment 2
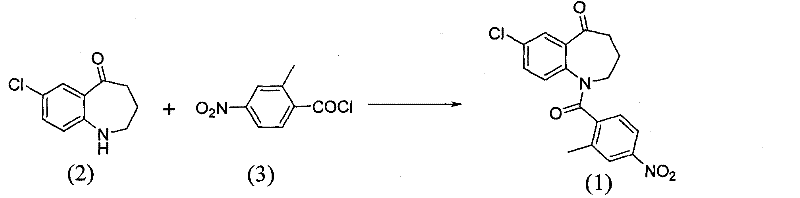
[0018] Add 24.08g (0.133mol) of 4-nitro-2-methylbenzoic acid into a 250ml single-necked flask, add 63.26g (4eq) of thionyl chloride, reflux for 5 hours, and distill off excess dichloride under reduced pressure. Sulfoxide, to obtain 26.6g of a brownish-yellow oil, which was solidified after condensation; dissolved in 10ml of acetonitrile for later use.
[0019] Add 20g (0.102mol) of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine into a 250ml three-necked flask, dissolve it with 100ml of acetonitrile, and then Add 4.08g (1eq) NaOH, stir, add dropwise 10ml of acetonitrile solution of 26.6g 4-nitro-2-methylbenzoyl chloride, react at room temperature, as the reaction progresses, the reaction solution gradually changes from dark green to light green At the same time, a large amount of white solids were precipitated. After 60 minutes, the dropwise addition was completed, and the reaction was stopped. The reaction solution was concentrated to dryness, and 200ml of dichloromethane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com