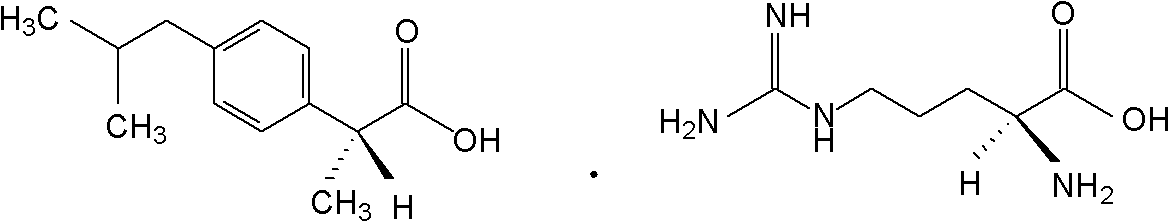
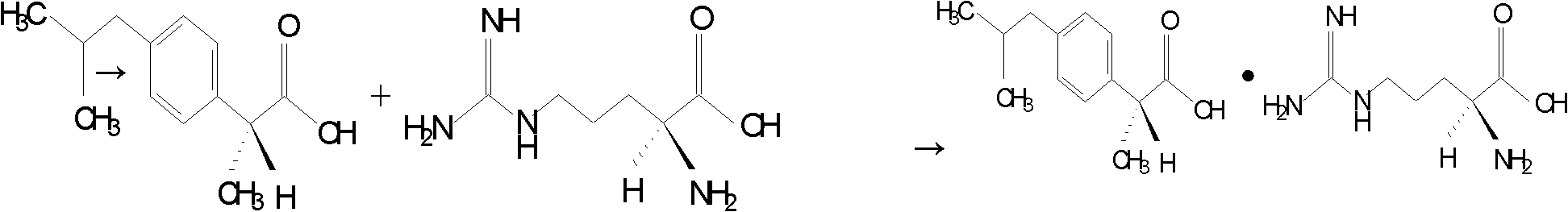
Preparation method of arginine dexibuprofen
A technology of dextrobuprofen and arginine, which is applied to the preparation of organic compounds, chemical instruments and methods, separation/purification of carboxylic acid compounds, etc., can solve the problem of poor control of dropping speed and flow rate, poor reaction, etc. Complete, related substances and other issues, to achieve the effect of correcting liver toxicity, increasing supply, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Dissolve 20.83g (0.101mol) of Dexibuprofen in 72ml of 95% (volume percent concentration) ethanol aqueous solution, heat to 55°C, and slowly and continuously add 17.42g (0.1mol) of L-arginine dropwise under stirring, After 10 minutes of dropwise addition, keep stirring at 55°C for 3 hours, let stand for 30 minutes, then cool to room temperature, filter with suction to obtain crystals, wash 3 times with 5ml of 95% (volume percent concentration) ethanol aqueous solution, and dry at 65°C for 6 hours to obtain white The crystal is 37.66g, the yield is 98.98%, and the melting point is 175°C-178°C.
[0035] The above-mentioned white crystals were subjected to elemental analysis, and the results of the main components were as follows: C: 60.04%, H: 8.51%, N: 14.71% (both by mass percentage), and the theoretical value of arginine Dexibuprofen: C: 59.97 %, H: 8.47%, N: 14.71% (both mass percentages) are consistent. It shows that the main component of the above product is D-α-met...
Embodiment 2
[0037] Dissolve 22.12g (0.107mol) of Dexibuprofen in 72ml of 95% (volume percent concentration) ethanol aqueous solution, heat to 50°C, and slowly and continuously add 17.42g (0.1mol) of L-arginine dropwise under stirring, After 15 minutes of dropwise addition, keep stirring at 50°C for 2 hours, let stand for 25 minutes, then cool to room temperature, filter with suction to obtain crystals, wash 3 times with 5ml of 95% (volume percent concentration) ethanol aqueous solution, and dry at 70°C for 4 hours to obtain white The crystal is 37.46g, the yield is 98.45%, and the melting point is 175.2°C-177.6°C.
[0038]The above-mentioned white crystals were subjected to elemental analysis, and the main component results were as follows: C: 60.12%, H: 8.48%, N: 14.77% (both by mass percentage), and the theoretical value of arginine Dexibuprofen: C: 59.97 %, H: 8.47%, N: 14.71% (both mass percentages) are consistent. It shows that the main component of the above product is D-α-methyl-4...
Embodiment 3
[0040] Dissolve 21.94g (0.106mol) of Dexibuprofen in 72ml of 95% (volume percent concentration) ethanol aqueous solution, heat to 60°C, and slowly and continuously add 17.42g (0.1mol) of L-arginine dropwise under stirring, After 20 minutes of dropwise addition, keep stirring at 60°C for 3.5 hours, let stand for 35 minutes, then cool to room temperature, filter with suction to obtain crystals, wash 3 times with 5ml of 95% (volume percent concentration) ethanol aqueous solution, and dry at 68°C for 5 hours to obtain 36.86 g of white crystals, the yield is 96.88%, and the melting point is 175.8°C to 178.2°C.
[0041] The above-mentioned white crystals are subjected to elemental analysis, and the main component results are as follows: C: 60.08%, H: 8.49%, N: 14.77% (both by mass percentage), and the theoretical value of arginine dextroibuprofen: C: 59.97 %, H: 8.47%, N: 14.75% (both mass percentages) match. It shows that the main component of the above product is D-α-methyl-4-(2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com