Preparation method for carbon-doped lithium stannate cathodal material for lithium batteries
A negative electrode material, lithium stannate technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problem of large irreversible capacity, and achieve the effect of avoiding rapid capacity decay, inhibiting agglomeration, and alleviating volume changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
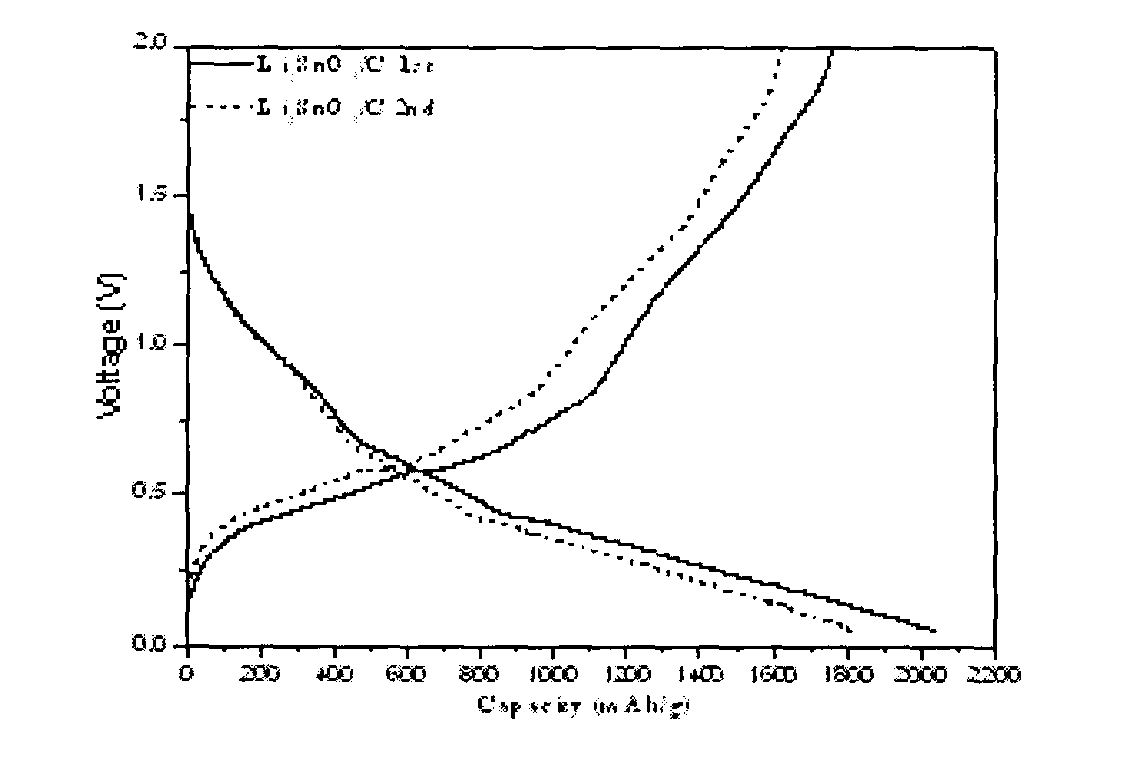
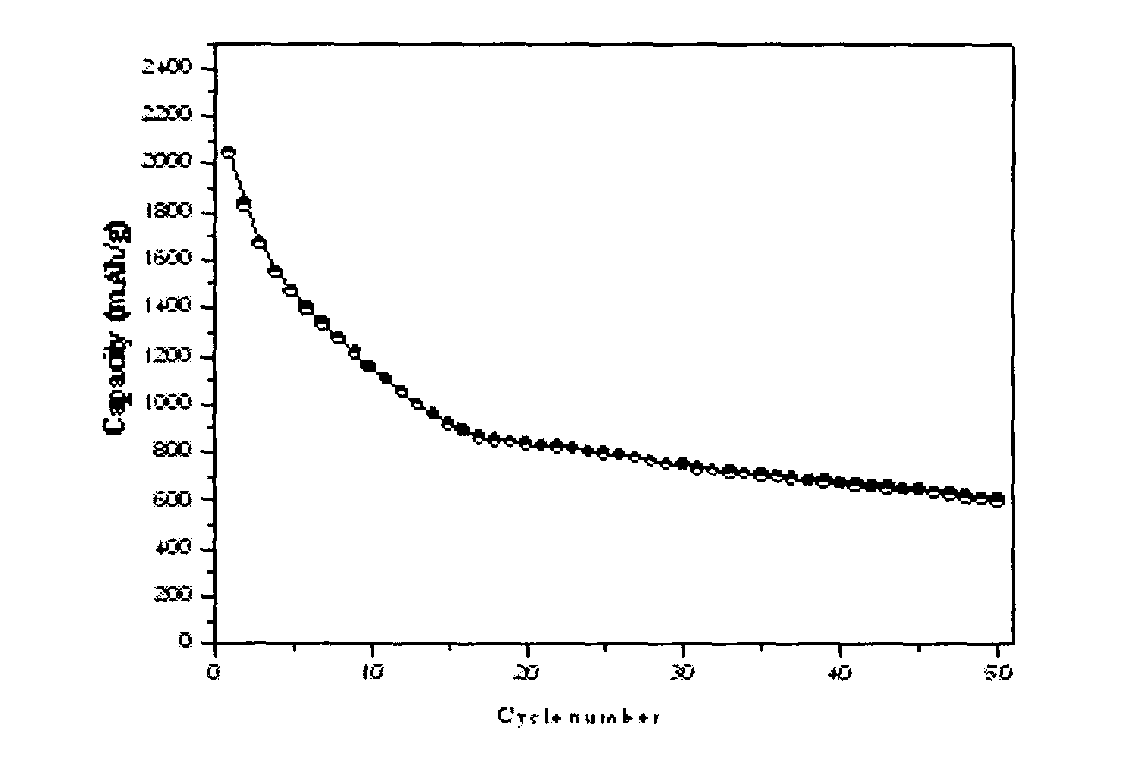
Image
Examples
Embodiment 1
[0019] (1) Hydrothermal process:
[0020] (a) A solution: SnCl 4 ·5H 2 O, LiOH and deionization were mixed in a ratio (molar ratio) of 1:6:1.67 to obtain A solution;
[0021] (b) Solution B: fully mix polyethylene glycol, absolute ethanol and deionized water at a ratio (molar ratio) of 1:98:35 to obtain Solution B;
[0022] (c) After the two are fully dissolved, under magnetic stirring, slowly drop the A solution into the B solution to completely dissolve it;
[0023] (d) Then, in the mixed solution, add 2.2mol / L glucose aqueous solution, and continue to stir until completely dissolved;
[0024] (e) Transfer the mixed solution into a 100ml stainless steel reaction kettle and keep it warm at 170°C for 24h.
[0025] (2) After centrifuging and washing the obtained material several times with deionized water and absolute ethanol, transfer it to a petri dish, put it in an oven, and dry it in an oven at 55°C to obtain a precursor;
[0026] (3) Heat treatment process: the precur...
Embodiment 2
[0029] (1) Hydrothermal process:
[0030] (a) A solution: SnCl 4 ·5H 2 O, LiOH and deionization were mixed in a ratio (molar ratio) of 1:6:1.67 to obtain A solution;
[0031] (b) Solution B: fully mix polyethylene glycol, absolute ethanol and deionized water at a ratio of 1:98:35 (molar ratio) to obtain Solution B;
[0032] (c) After the two are fully dissolved, under magnetic stirring, slowly drop the A solution into the B solution to completely dissolve it;
[0033](d) Then, in the mixed solution, add 2.2mol / L glucose aqueous solution, and continue to stir until completely dissolved;
[0034] (e) Move the mixed solution into a 100ml stainless steel reactor and keep it warm at 173°C for 22h.
[0035] (2) After centrifuging and washing the obtained material several times with deionized water and absolute ethanol, transfer it to a petri dish, put it in an oven, and dry it in an oven at 58°C to obtain a precursor;
[0036] (3) Heat treatment process: the precursor was sinte...
Embodiment 3
[0039] (1) Hydrothermal process:
[0040] (a) A solution: SnCl 4 ·5H 2 O, LiOH and deionization were mixed in a ratio (molar ratio) of 1:6:1.67 to obtain A solution;
[0041] (b) Solution B: fully mix polyethylene glycol, absolute ethanol and deionized water at a ratio (molar ratio) of 1:98:35 to obtain Solution B;
[0042] (c) After the two are fully dissolved, under magnetic stirring, slowly drop the A solution into the B solution to completely dissolve it;
[0043] (d) Then, in the mixed solution, add 2.2mol / L glucose aqueous solution, and continue to stir until completely dissolved;
[0044] (e) Transfer the mixed solution into a 100ml stainless steel reaction kettle and keep it warm at 176°C for 20h.
[0045] (2) After centrifuging and washing the obtained substance several times with deionized water and absolute ethanol, transfer it to a petri dish, put it in an oven, and dry it in an oven at 59°C to obtain a precursor;
[0046] (3) Heat treatment process: the precu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com