Bone targeting vector and medicament
A bone targeting and drug technology, applied in the field of targeted drugs of drugs, can solve the problems of weak tumor killing effect and low drug loading, and achieve the effects of easy control of conditions, good biocompatibility and fast response speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
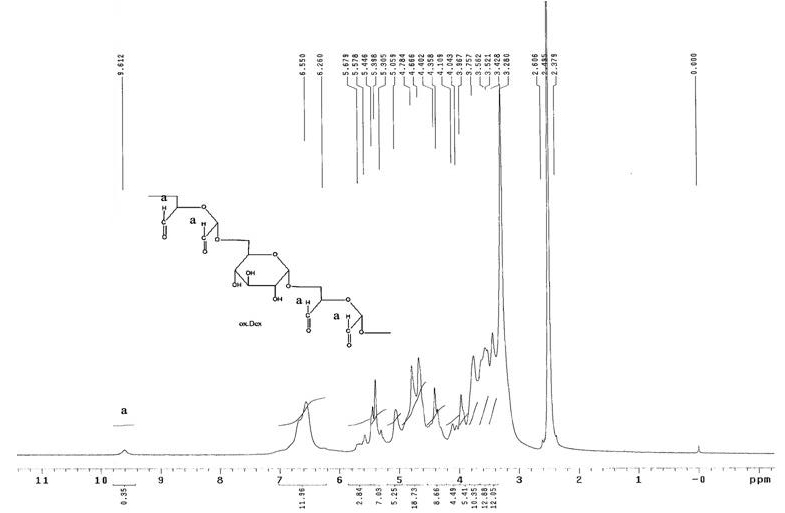
[0034] Example 1: Synthesis of oxidized dextran
[0035] Weigh 10.0g of dextran (T-40) and 13.5g of sodium periodate, and dissolve them in 200ml and 150ml of pH4.4 phosphate buffer solution respectively to obtain dextran solution and sodium periodate solution. Add the sodium solution into the dextran solution, stir at room temperature at 1500r / min in the dark for 4.5 hours, immediately add 4.5ml of glycerol, continue stirring at room temperature at 1500r / min for 15 minutes, place the reaction mixture in a dialyzer with a cut-off molecular weight of 3500 The bag was dialyzed at 4°C for 48 hours with distilled water as the medium, and the dialysate was freeze-dried to obtain 5.1 g of oxidized dextran. oxidized dextran 1 HNMR spectrum see figure 1 , 1 HNMR (600MHz, DMSO): δ H =9.61 ppm is the peak generated by aldehyde protons.
Embodiment 2
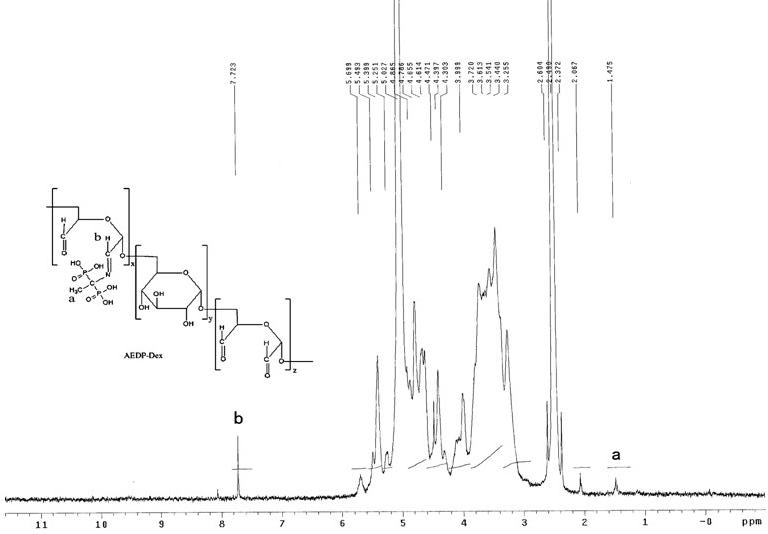
[0036] Example 2: Synthesis of Bisphosphonate Oxidized Dextran Bone Targeting Drug Carrier
[0037] Take 0.6mmol AEDP and dissolve it in 10ml distilled water. Weigh 100 mg of oxidized dextran (with an aldehyde group content of about 1.0 mmol), dissolve it in 5 ml of distilled water, add AEDP solution to it, incubate at 4 °C for 24 h, place the reaction mixture in a dialysis bag with a cut-off molecular weight of 3500, and distilled water The medium was dialyzed at 4°C in the dark for 48 hours to remove unbound AEDP, and the dialysate was freeze-dried to obtain a white powdery bone-targeting drug carrier.
[0038] 1 HNMR (600MHz, DMSO): δ H =1.47 ppm multiple peaks appear, should be -CH on AEDP 3 introduce. δ H The peak at =7.72 ppm is the peak generated by the Schiffer base structure -HC=N-proton. (see attached figure 2 )
Embodiment 3
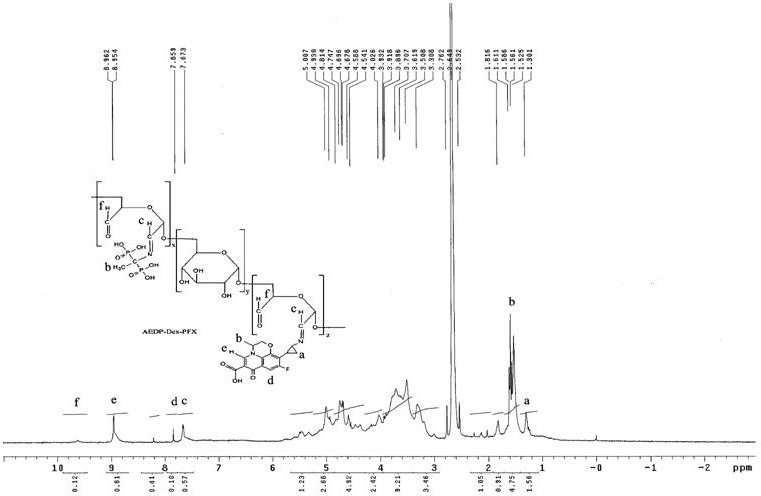
[0039] Example 3: Synthesis of bone-targeted pazufloxacin AEDP-Dex-PFX
[0040] Step 1 is the same as in Example 1.
[0041] Step 2: Take 0.6mmol AEDP and dissolve it in 10ml distilled water. Weigh 100mg of oxidized dextran (with an aldehyde content of about 1.0mmol), dissolve it in 5ml of distilled water, add the AEDP solution, and incubate at 4°C for 24h.
[0042] Step 3: Weigh 20 mg of PFX: dissolve it in 10 ml of distilled water with equimolar hydrochloric acid, add it to the above reaction solution, stir with magnetic force, and incubate at 4°C in the dark for 24 hours. Finally, the reaction solution was passed through a Sephadex G-50 gel column, and the first fraction was collected and freeze-dried to obtain a pale yellow powder product bone-targeted pazufloxacin, with a drug loading of 3.38% and an in vitro HA adsorption rate of 89.12%. %.
[0043] 1 HNMR (600MHz, DMSO): δ H =1.30 is the methylene proton peak of cyclopropane on PFX; δ H The multiple peaks at =1.53...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com