Tilmicosin liposome injection and preparation method thereof
A technology of tilmicosin and injection, which is applied in the field of tilmicosin liposome injection and its preparation, can solve the problems of negative cardiac effect, few injections, and large side effects, so as to reduce toxic and side effects , The preparation process is simple, and the effect of increasing the blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
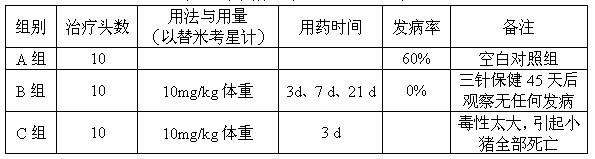
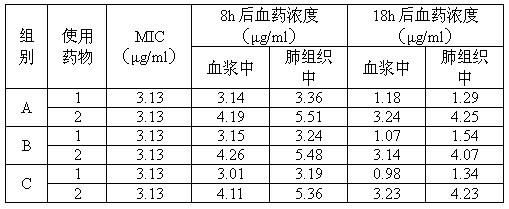
[0015] For tilmicosin liposome injection, each 100ml injection contains the following components: 20ml propylene glycol, 3g soybean lecithin, 1.5g cholesterol, 1g Span 80, 1g Tween-80, 5g tilmicosin, the balance For injection water.
[0016] Dosing: Take 20ml of propylene glycol, add 3g of soybean lecithin, 1.5g of cholesterol, 1g of Span 80, 1g of Tween-80, 5g of tilmicosin, heat until dissolved, then add water to 100ml, stir while adding, until into an emulsified solution, added to a colloid mill to grind to a fine liquid, potted and sterilized to obtain tilmicosin liposome injection for animals.
[0017] Quality inspection: the obtained injection is a light yellow milky liquid; the content of tilmicosin is 5g / 100ml, and all inspections are in line with the provisions of the Chinese Veterinary Pharmacopoeia on emulsions, and the injection is valid for 2.43 years.
Embodiment 2
[0019] The tilmicosin liposome injection contains the following components per 100ml injection: 20ml oleic acid, 3g soybean lecithin, 1.5g cholesterol, 2g Tween-80, 6g tilmicosin, and the balance is water for injection.
[0020] Dosing: Take 20ml of oleic acid, add 3g of soybean lecithin, 1.5g of cholesterol, 2g of Tween-80, and 6g of tilmicosin, heat until dissolved, then add water to 100ml, and stir while adding until it becomes a milky solution. Add it into a colloid mill to grind to a fine liquid, potting and sterilizing to obtain the tilmicosin liposome injection for veterinary use.
[0021] Quality inspection: the obtained injection is a light yellow milky liquid; the content of tilmicosin is 6g / 100ml, and all inspections are in line with the provisions of the Chinese Veterinary Pharmacopoeia on emulsions, and the validity period of the injection is 2.36 years.
Embodiment 3
[0023] For tilmicosin liposome injection, every 100ml injection contains the following components: 20ml propylene glycol, 4g soybean lecithin, 2g cholesterol, 2g glycerol monostearate, 8g tilmicosin, and the balance is water for injection.
[0024] Dosing: Take 20ml of propylene glycol, add 4g of soybean lecithin, 2g of cholesterol, 2g of glyceryl monostearate, and 8g of tilmicosin, heat until dissolved, then add water to 100ml, and stir while adding until it becomes a milky solution. Add it into a colloid mill to grind to a fine liquid, potting and sterilizing to obtain the tilmicosin liposome injection for veterinary use.
[0025] Quality inspection: the obtained injection is a light yellow milky liquid; the content of tilmicosin is 8g / 100ml, and all inspections are in line with the provisions of the Chinese Veterinary Pharmacopoeia on emulsions, and the injection is valid for 2.47 years.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com