Detection method of haemostasis medicine
A detection method and drug technology, which is applied in the detection of capsules and the detection of hemostatic drugs, can solve the problems of human body and environmental hazards, and the undeveloped TLC identification method, liquid phase detection method, content detection method, etc., to reduce harm and purify drugs Market and order, maintenance of security and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
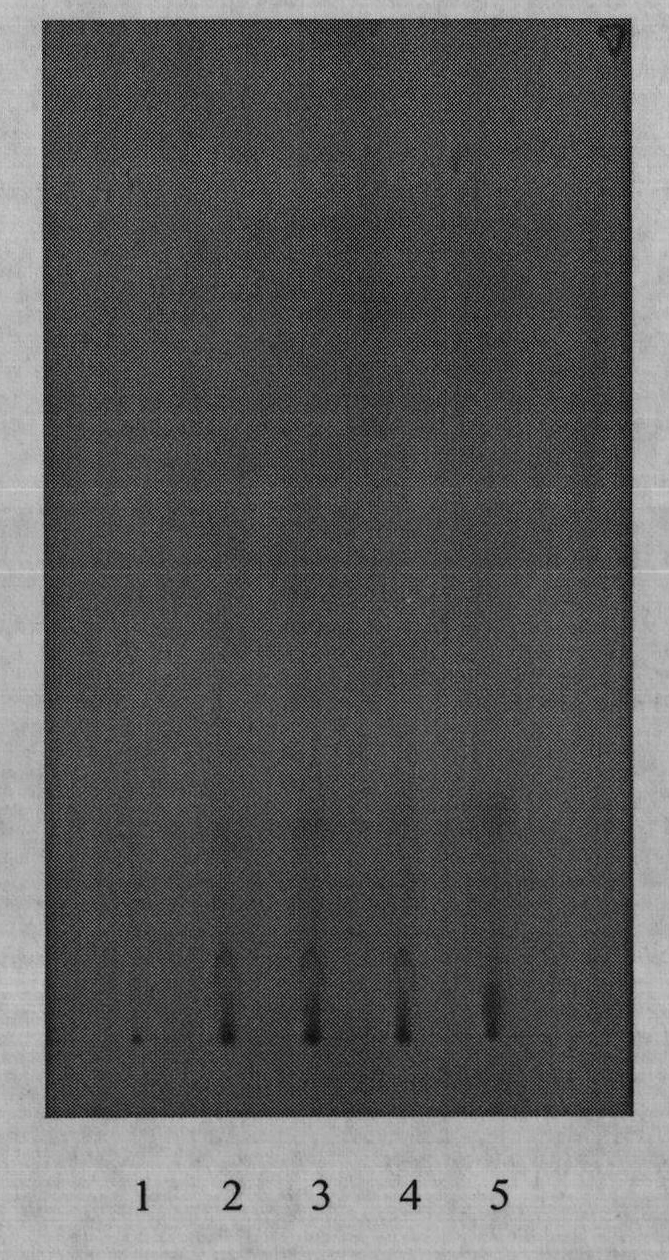
[0067] Example 1 TLC Identification of Myrrh in Zhikang Capsules
[0068] 1. Test conditions: double-slot expansion cylinder (Shanghai Xinyi); silica gel G (for thin-layer chromatography, Qingdao Ocean Chemical Factory); sodium carboxymethyl cellulose (Sinopharm Chemical Reagent Co., Ltd.); self-made thin-layer plate, thickness 0.3mm. The rest of the reagents were analytically pure.
[0069] 2. Test procedure: Take 3g of the contents of Zhikang Capsules (provided by Xi’an Qianhe Pharmaceutical Co., Ltd.), put them in a stoppered Erlenmeyer flask, add 50ml of absolute ethanol, ultrasonicate for 60 minutes, filter, and evaporate the filtrate to dryness. Add 50ml of water to dissolve, filter, extract the filtrate with ether 3 times, 20ml each time, collect and combine the ether solution, evaporate to dryness, add 1ml of petroleum ether (60-90°C) to the residue to dissolve, and use it as the test solution. Take another 0.3 g of myrrh reference medicinal material, and prepare t...
Embodiment 2
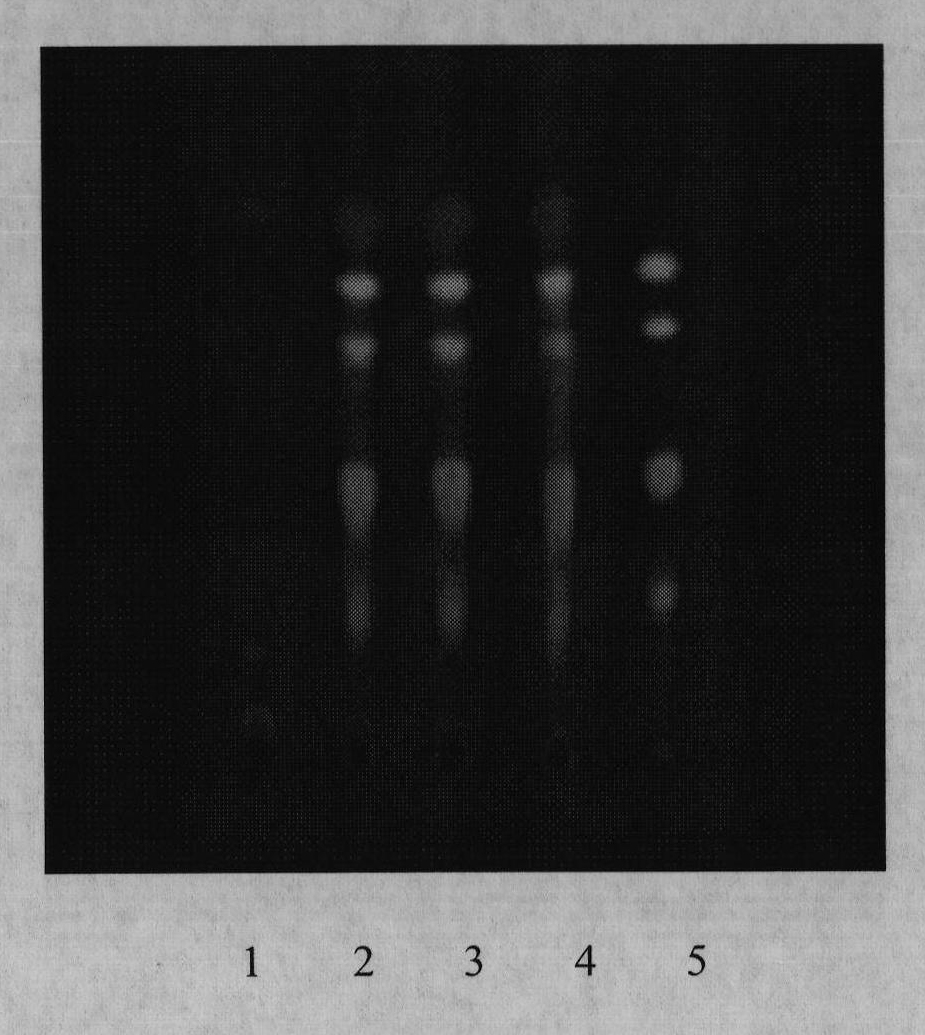
[0072] Example 2 TLC Identification of Coptis Rhizoma in Zhikang Capsules
[0073] 1. Test conditions: double-slot expansion cylinder (Shanghai Xinyi); silica gel G (for thin-layer chromatography, Qingdao Ocean Chemical Factory); sodium carboxymethyl cellulose (Sinopharm Chemical Reagent Co., Ltd.); self-made thin-layer plate, thickness 0.3mm. The rest of the reagents were analytically pure.
[0074] 2. Test procedure: take 3 g of the content of Zhikang Capsules (provided by Xi’an Qianhe Pharmaceutical Co., Ltd.), add 20 ml of 1% hydrochloric acid methanol solution, ultrasonically treat for 20 minutes, filter, and concentrate the filtrate to 1 ml as the test sample solution. Take another 0.1 g of Coptis rhizome reference drug, add 5 ml of 1% hydrochloric acid methanol solution, filter, and use the filtrate as the reference drug solution. Then take the negative sample without Coptis rhizome prepared according to the prescription ratio and process, and prepare the negative ...
Embodiment 3
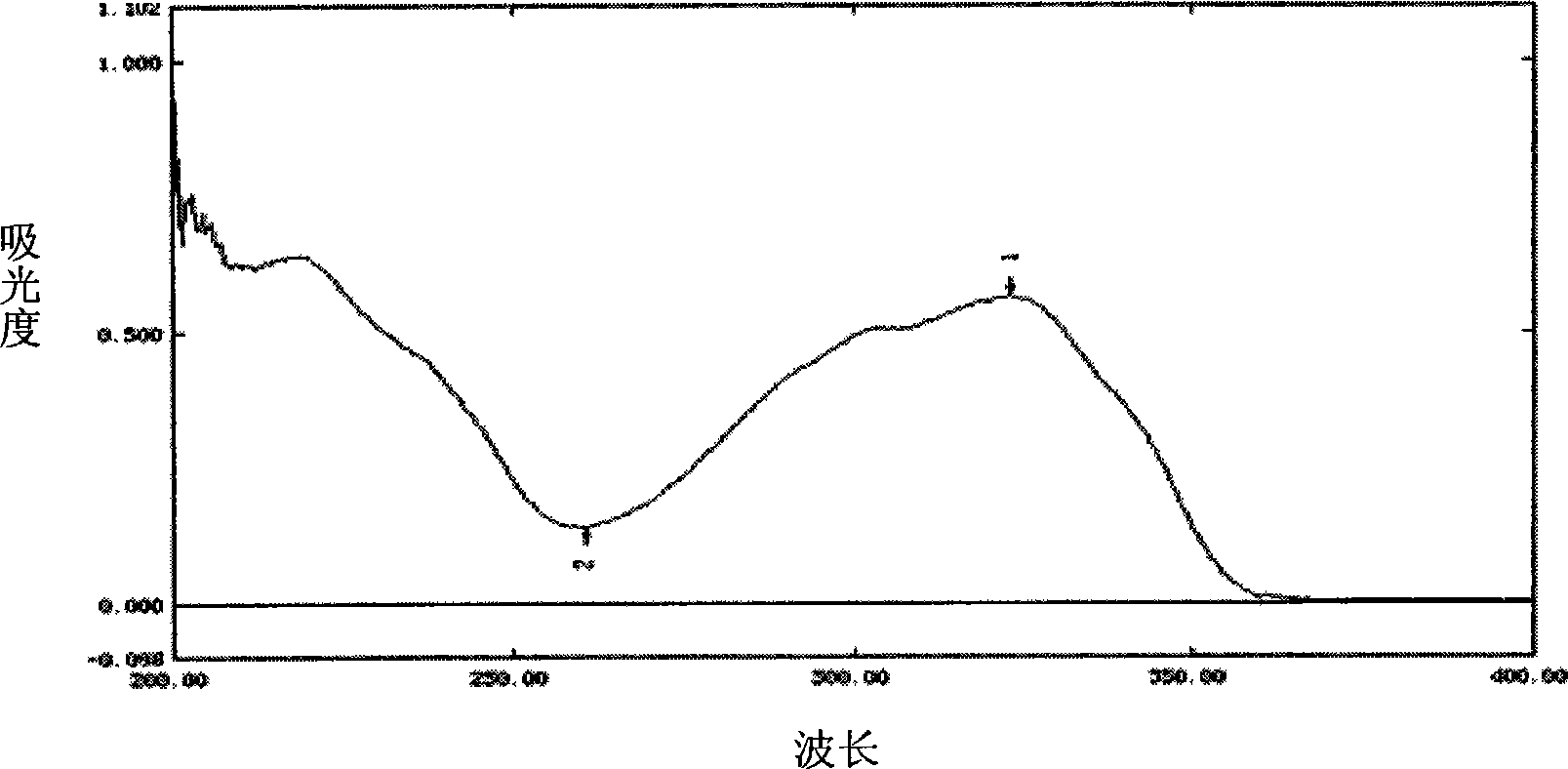
[0075] Example 3 Determination of rhubarb in rhubarb by liquid chromatography
[0076] 1. Detection method
[0077] Determined with reference to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 Edition).
[0078] 1. Chromatographic conditions and system suitability test
[0079] Octadecylsilane bonded silica gel is used as filler; methanol-water (36:64) is used as mobile phase; the detection wavelength is 320nm; the number of theoretical plates should not be less than 2000 based on the calculation of phedin peak.
[0080] 2. Preparation of reference solution
[0081] Take an appropriate amount of rhubarb reference substance, weigh it accurately, add methanol to make a solution containing 10 μg per 1 ml, and obtain it.
[0082] 3. Preparation of the test solution
[0083] Take the contents of Zhikang Capsules (provided by Xi’an Qianhe Pharmaceutical Co., Ltd.), grind finely, take 0.5g, accurately weigh it, put it in a 50ml measuring bott...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com