Chitosan aminoethyl quaternary ammonium salt derivative and preparation method thereof
A technology of aminoquaternary ammonium and aminoethyl, which is applied in the field of chitosan aminoethylquaternary ammonium salt derivatives and its preparation, can solve the problems that there are no reports of chitosan aminoethylquaternary ammonium salt derivatives, etc. Achieve good solubility, expand application fields, and improve biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 derivative 1
[0030] 1.0 grams of aminoethyl chitosan and 3.20 grams of sodium iodide were added to 40 mL of N-methylpyrrolidone, 8 mL of 15% sodium hydroxide was added dropwise to it under stirring, stirred at 60° C. for 20 minutes, 8 mL of methyl iodide was added, and 60 Reaction at ℃ for 60 minutes, precipitation with ethyl ether, washing with ether, dissolving in 50 mL of 10% sodium chloride solution, shaking on a shaker for 18 hours, filtering, dialysis, concentration and freeze-drying to obtain a white cotton-like solid, which is chitosan aminoethyl methyl Base quaternary ammonium salt, that is, derivative 1, see Table 1 for the structural formula.
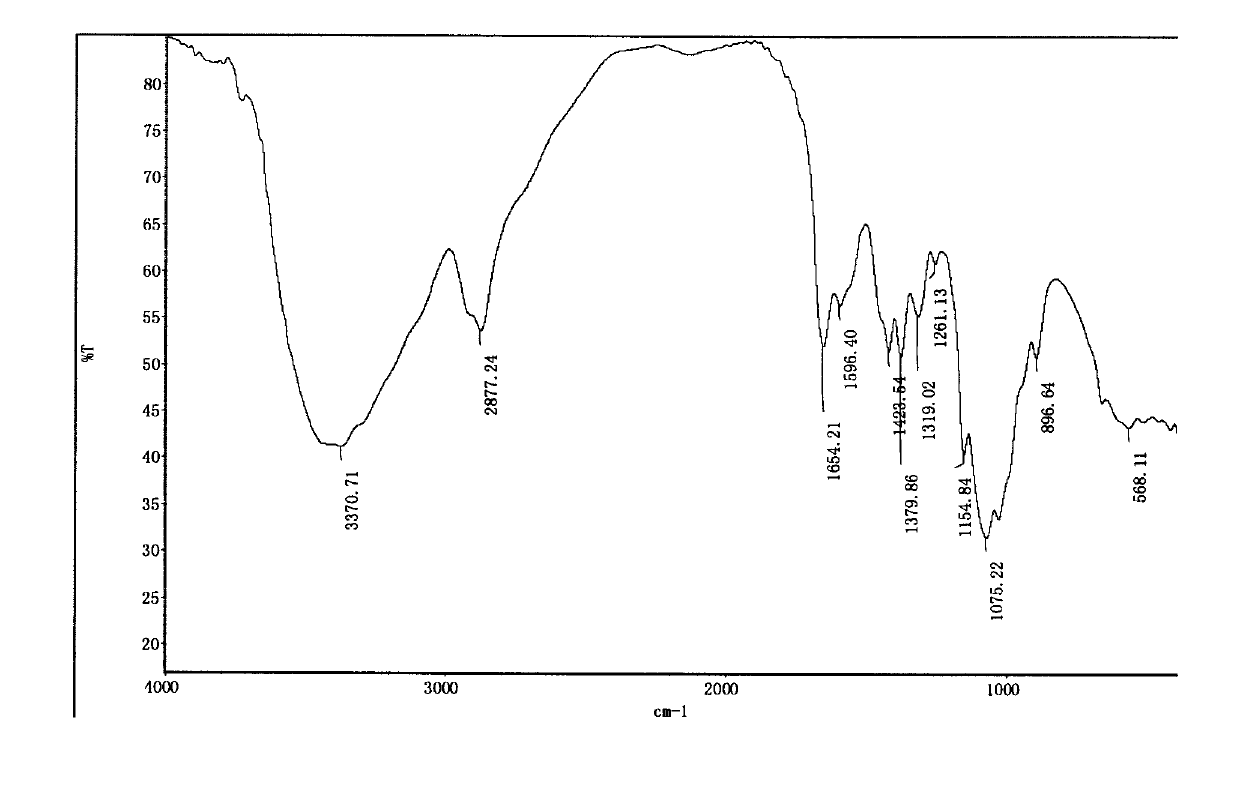
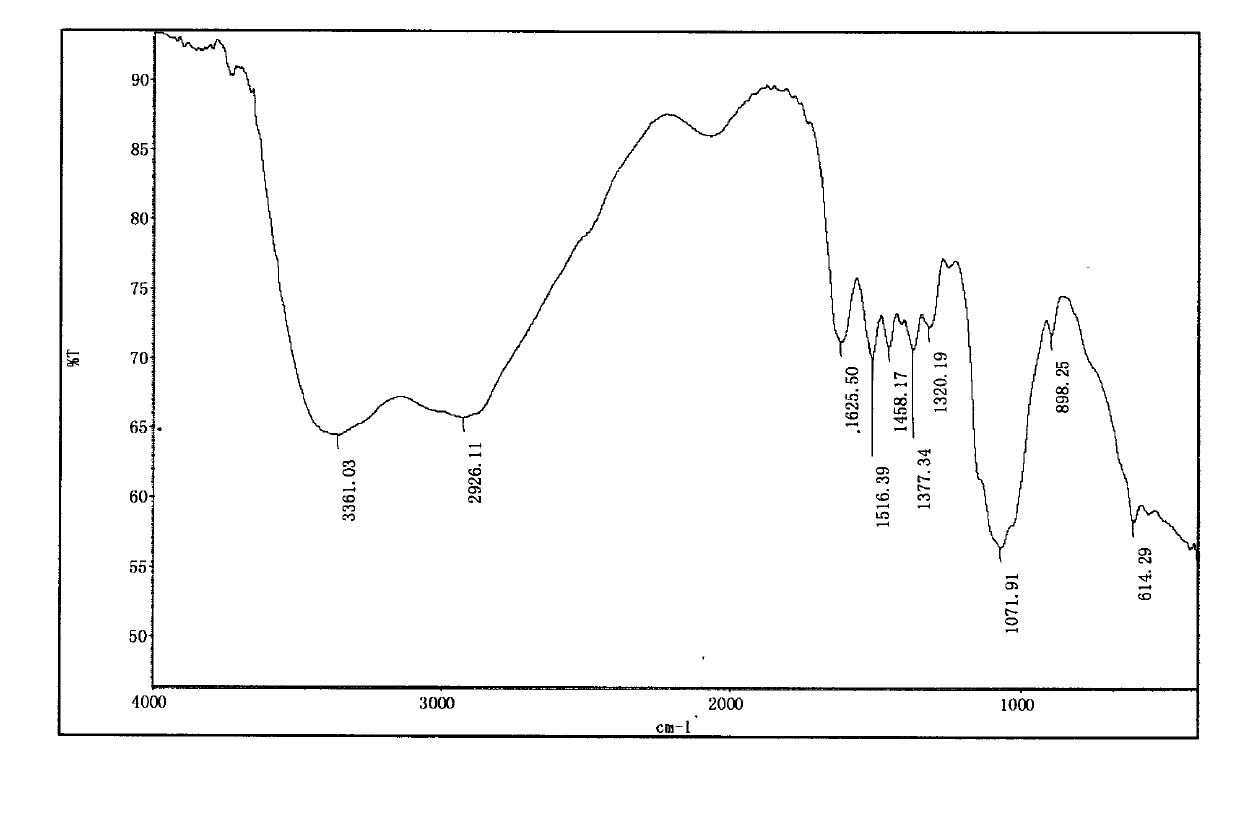
[0031] Infrared spectrum shows: the infrared spectrum of chitosan derivative 1 ( image 3 ) and the infrared spectrum of aminoethyl chitosan ( figure 2 ) compared to 3030, 2943, 2870cm -1 The hydrocarbon stretching vibration peak of the methyl group is enhanced, and it moves to the...
Embodiment 2
[0034] The preparation of embodiment 2 derivative 2
[0035] Add 3.0 g of aminoethyl chitosan to 120 mL of distilled water, stir to dissolve, add 1.8 mL of n-butyraldehyde dropwise, stir at room temperature for 2 hours, slowly add 38 mL of 10% sodium borohydride dropwise, continue the reaction for 2 hours, and precipitate with acetone after the reaction , the obtained solid was placed and dried at 60° C. to obtain N-substituted aminoethyl chitosan.
[0036] Add 1 gram of N-substituted aminoethyl chitosan and 3.20 grams of sodium iodide to 40 mL of N-methylpyrrolidone, add 8 mL of 15% sodium hydroxide dropwise under stirring, stir at 60°C for 20 minutes, and add 8 mL of iodine Methane, reacted at 60°C for 60 minutes, precipitated with ethanol and ether, washed with ether, dissolved in 50mL of 10% sodium chloride solution, shaken on a shaker for 18 hours, filtered, dialyzed, concentrated and freeze-dried to obtain a white cotton-like solid, which is chitosan ammonia Ethylmethyl...
Embodiment 3
[0038] The preparation of embodiment 3 derivative 3
[0039] Add 3.0 g of aminoethyl chitosan to 120 mL of distilled water, stir and dissolve, then add 1.8 mL of benzaldehyde dropwise, stir at room temperature for 2 hours, slowly add 38 mL of 10% sodium borohydride dropwise, continue the reaction for 2 hours, and precipitate with acetone after the reaction. The obtained solid was placed and dried at 60° C. to obtain N-substituted aminoethyl chitosan.
[0040] Add 1 gram of N-substituted aminoethyl chitosan and 3.20 grams of sodium iodide to 40 mL of N-methylpyrrolidone, add 8 mL of 15% sodium hydroxide dropwise under stirring, stir at 60°C for 20 minutes, and add 8 mL of iodine Methane, reacted at 60°C for 60 minutes, precipitated with ethanol and ether, washed with ether, dissolved in 50mL of 10% sodium chloride solution, shaken on a shaker for 18 hours, filtered, dialyzed, concentrated and freeze-dried to obtain a white cotton-like solid; namely, chitosan ammonia Ethylmethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com