Preparation method of barium oxalate hydrate microcrystal with firewood morphology
A technology of hydrated barium oxalate and morphology, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of complex preparation methods and low product quality, and achieve the effects of low preparation cost, high product yield and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
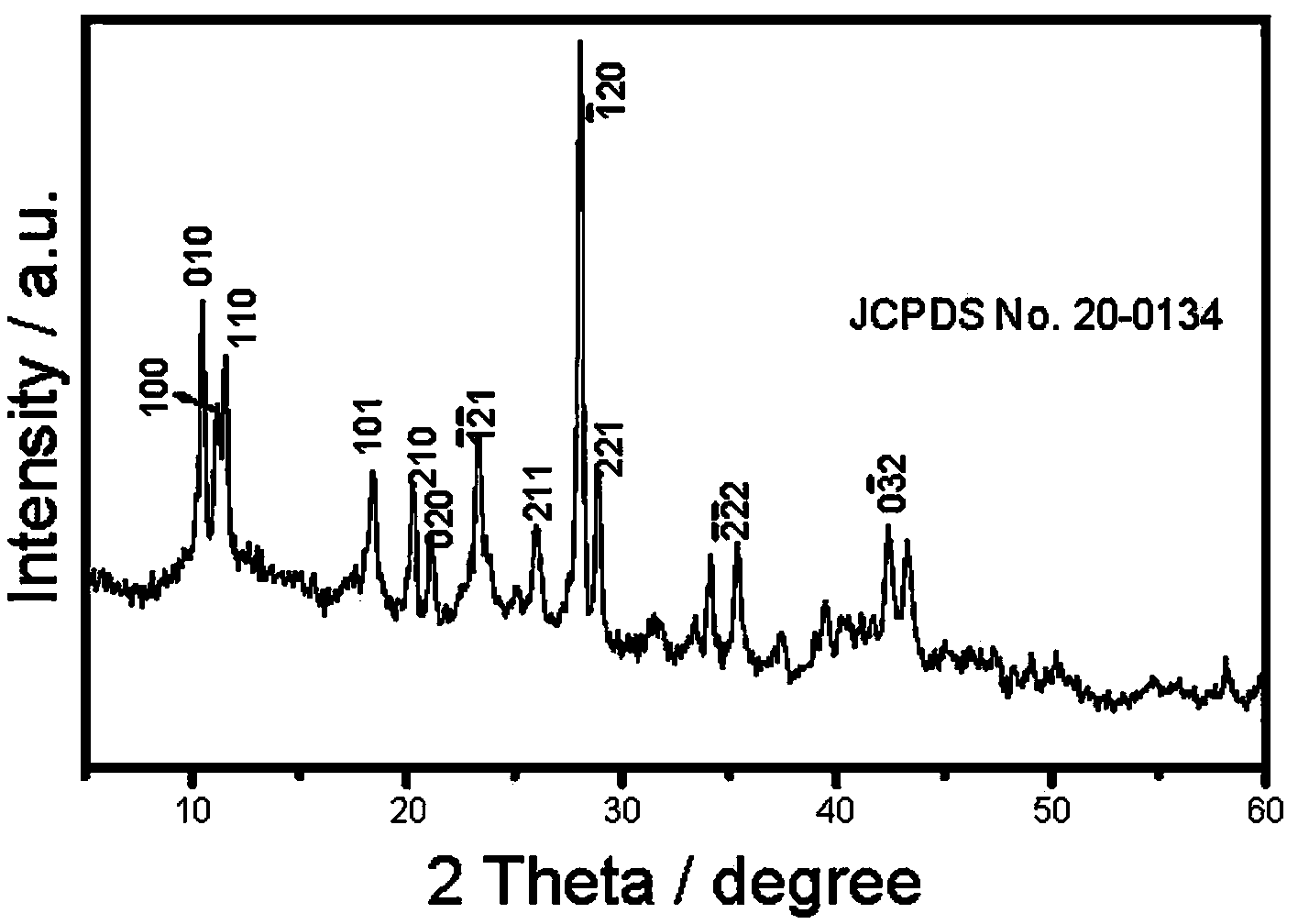
Image
Examples
Embodiment 1
[0024] Slowly add the 0.05 mol / L barium chloride solution dropwise to the 0.05 mol / L sodium oxalate solution at room temperature. The amount of the mixed solution added is based on the molar ratio of barium chloride / sodium oxalate = 1:0.5, and the reaction is stirred for 20 minutes at a stirring speed of 70 rpm. The resulting mixed solution was subjected to an aging reaction, the aging reaction temperature was 25 ° C, and the aging reaction time was 12 hours. After the aging reaction, the product obtained by the reaction was filtered and washed, put into an oven, and dried for 4 hours at 40 ° C to obtain a hydrated barium oxalate product.
Embodiment 2
[0026] Slowly add the 0.05 mol / L barium chloride solution dropwise to the 0.05 mol / L sodium oxalate solution at room temperature. The amount of the mixed solution is based on the molar ratio of barium chloride / sodium oxalate = 1:2, and the reaction is stirred for 20 minutes at a stirring speed of 70 rpm. The resulting mixed solution was subjected to an aging reaction, the aging reaction temperature was 25 ° C, and the aging reaction time was 12 hours. After the aging reaction, the product obtained by the reaction was filtered and washed, put into an oven, and dried for 4 hours at 40 ° C to obtain a hydrated barium oxalate product.
Embodiment 3
[0028] Slowly add the 0.05 mol / L barium chloride solution dropwise to the 0.05 mol / L potassium oxalate solution at room temperature. The amount of the mixed solution is based on the molar ratio of barium chloride / potassium oxalate=1:0.5, and the reaction is stirred for 20 minutes at a stirring speed of 90 rpm. The obtained mixed solution was subjected to an aging reaction, the aging reaction temperature was 25 ° C, and the aging reaction time was 8 hours. After the aging reaction, the product obtained by the reaction was filtered and washed, then put into an oven, and dried for 3 hours at 40°C to obtain a hydrated barium oxalate product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com