Novel method for synthesizing polypeptide containing aspartic acid-arginine and derivate units
A technology of aspartic acid and arginine, applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve problems such as failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
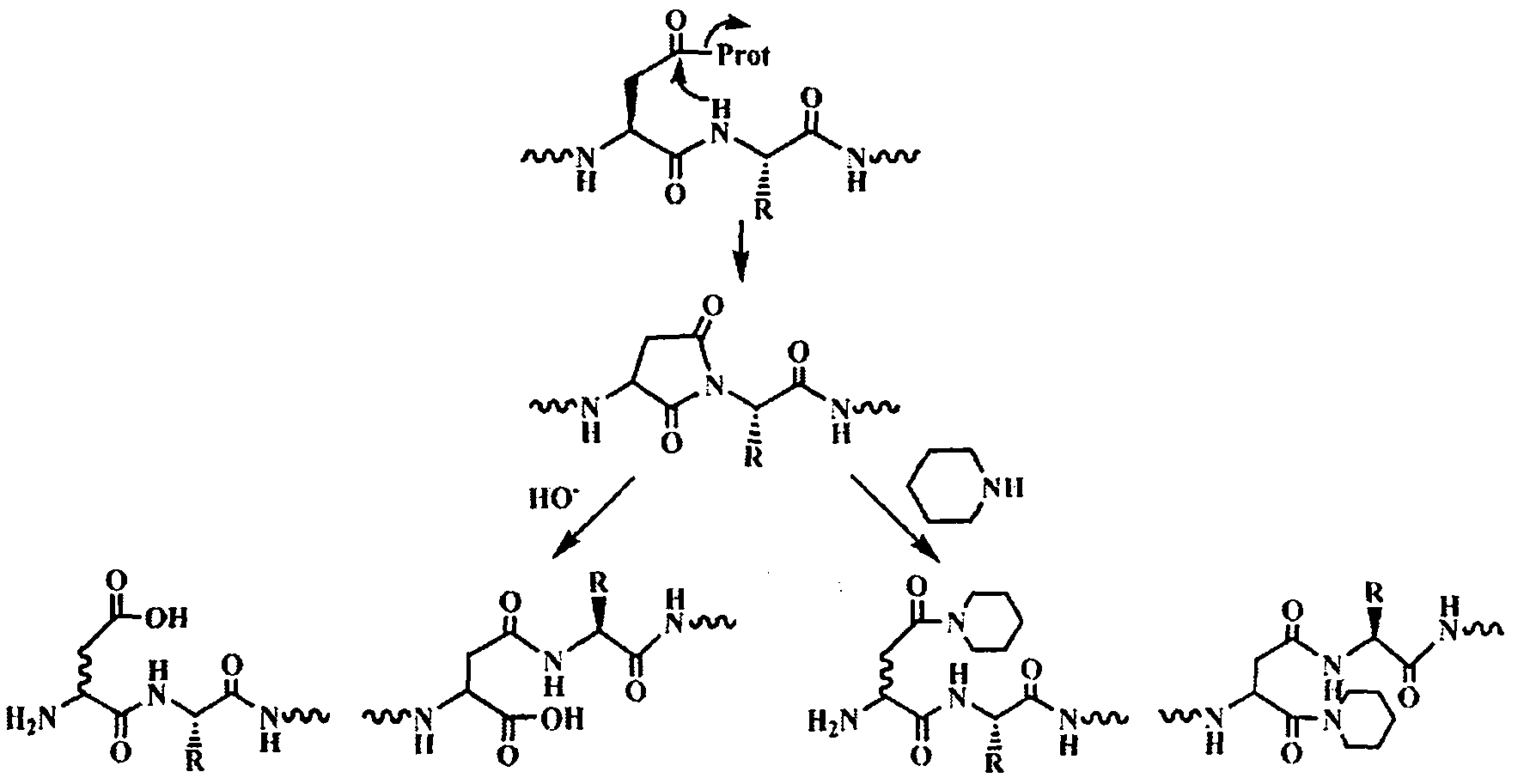
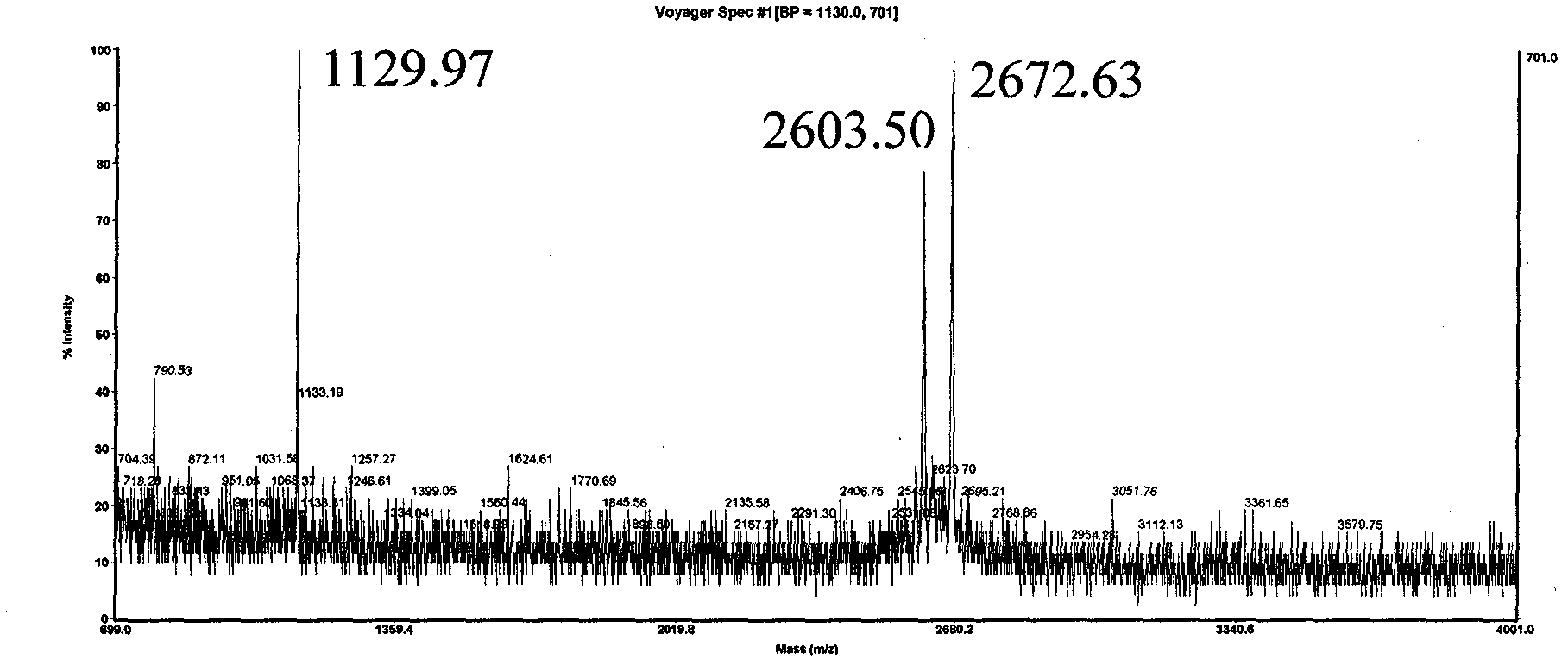
[0026] The sequence of polypeptide 3 is Biotin-Gly-Asp-[Arg(Me) 2 ]-Gly-Gly-Phe-Gly-Pro-Gly-Lys-Met-Asp-Ser-Arg-Gly-Glu-His-Arg-Gln-Asp-Arg-Arg-Glu-Arg-Pro-Tyr-OH. Since Asp-[Arg(Me) 2 ] unit, the Asp residue is extremely prone to the side reaction of forming asparagine, which leads to the inability to prepare the target polypeptide molecule by traditional methods. Adopt solid-phase synthesis sequence Biotin-Gly-Asp on CTC resin, cut 1 hour in 1 volume % trifluoroacetic acid / dichloroethane, filtrate is added to 10 times amount of petroleum ether / anhydrous ether (1: 1, v / In v), the polypeptide is precipitated, centrifuged and dried to obtain the crude Biotin-Gly-Asp(OtBu)-OH. Combine this fragment with H-[Arg(Me) 2 ]-Gly-Gly-Phe-Gly-Pro-Gly-Lys(Boc)-Met-Asp(OtBu)-Ser(tBu)-Arg(Pbf)-Gly-Glu(OtBu)-His(Trt)-Arg( Pbf)-Gln(Trt)-Asp(OtBu)-Arg(Pbf)-Arg(Pbf)-Glu(OtBu)-Arg(Pbf)-Pro-Tyr(tBu)-King resin for fragment condensation to get the whole target The sequence of the polypeptide...
Embodiment 2
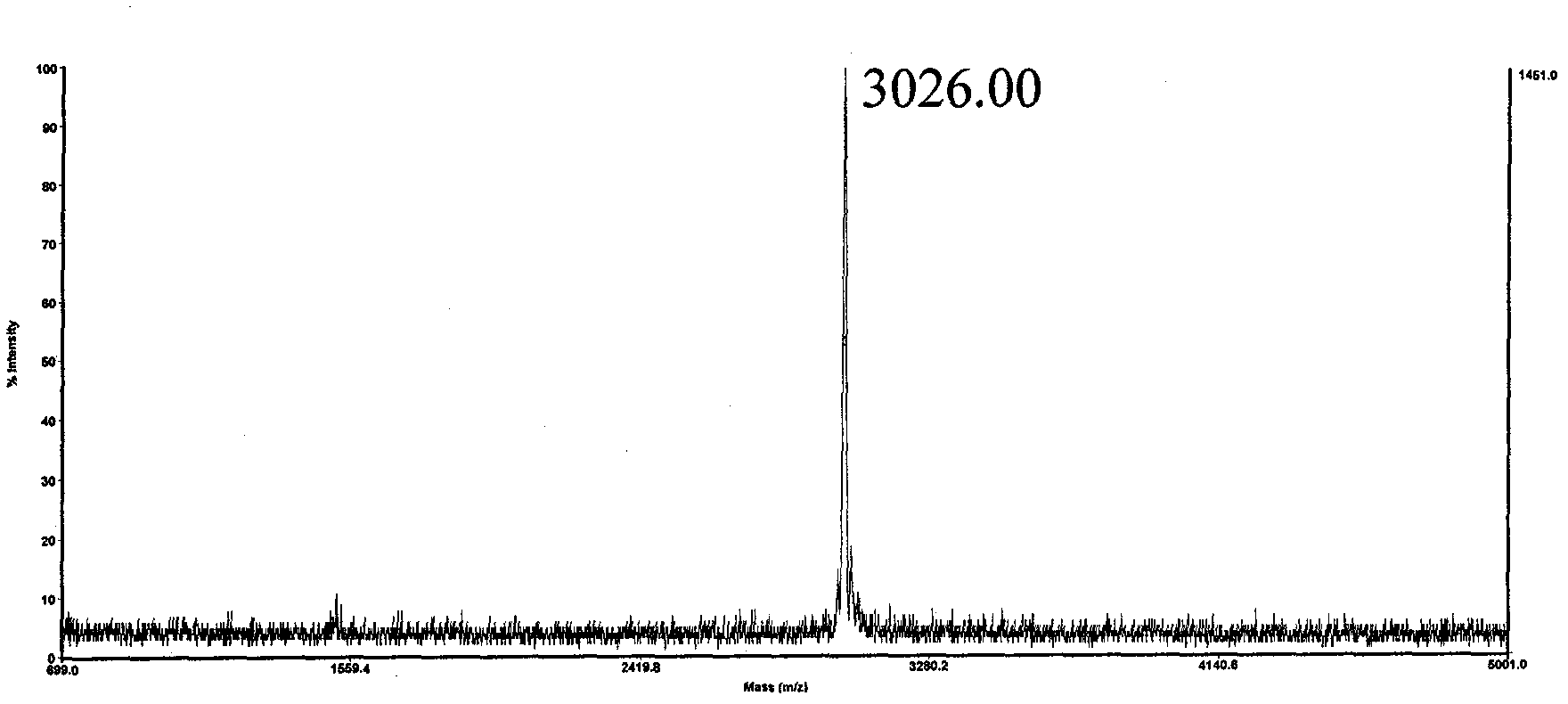
[0028] The sequence of polypeptide 4 is Biotin-Gly-Asp-Arg-Gly-Gly-Phe-Gly-Pro-Gly-Lys-Met-Asp-Ser-Arg-Gly-Glu-His-Arg-Gln-Asp-[Arg( Me) 2 ]-ArgGlu-Arg-Pro-Tyr-OH. Since Asp-[Arg(Me) 2 ] unit, the Asp residue is extremely prone to the side reaction of forming asparagine, and the target polypeptide molecule cannot be prepared by traditional methods. Solid-phase synthesis of the sequence Biotin-Gly-Asp-Arg-Gly-Gly-Phe-Gly-Pro-Gly-Lys-Met-Asp-Ser-Arg-Gly-Glu-His-Arg-Gln-Asp on CTC resin -OH, cleaved in 1 volume% trifluoroacetic acid / dichloroethane for 1 hour, the filtrate was added to 10 times the amount of petroleum ether / anhydrous ether (1:1, v / v) to precipitate the polypeptide, and dried after centrifugation. That is to obtain Biotin-Gly-Asp(OtBu)-Arg(Pbf)-Gly-Gly-Phe-Gly-Pro-Gly-Lys(Boc)-Met-Asp(OtBu)-Ser(tBu)-Arg(Pbf)- Crude Gly-Glu(OtBu)-His(Trt)-Arg(Pbf)-Gln(Trt)-Asp(OtBu)-OH. Combine this fragment with H-[Arg(Me) 2 ]-Arg(Pbf)-Glu(OtBu)-Arg(Pbf)-Pro-Tyr(tBu)-Wang res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com