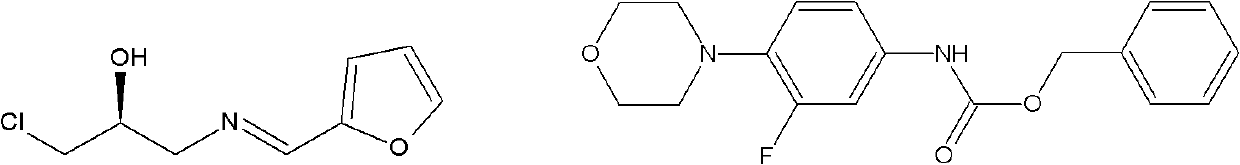
Intermediate for preparing linezolid
A technology of linezolid and furfuralimine, which is applied in the field of drug synthesis and can solve problems such as environmental damage, high toxicity, and loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the preparation of furfural imine
[0088]
[0089] Add 20 g of furfural and 32 ml of ammonia water into the reaction flask, stir, and react overnight at room temperature. Suction filter the reaction product, wash the filter cake with a small amount of water, and dry to obtain 19.2 g of furfural imine.
Embodiment 2
[0090] Embodiment 2: Preparation of (S)-1-chloro-3-[(furan-2-methylene)amino]propan-2-ol (structural formula II)
[0091]
[0092] In the reaction flask, 19.2 g of furfural imine prepared in Example 1, 120 ml of methyl tert-butyl ether, and 16 ml of S-epichlorohydrin were added. Stir for 30 minutes, slowly heat to reflux, react for 6 hours, cool to room temperature, concentrate to dryness under reduced pressure at 40°C, add 30ml of methyl tert-butyl ether to dissolve, crystallize in the refrigerator, filter with suction, filter cake with cold methyl tert-butyl ether After washing with butyl ether and vacuum drying to constant weight, 29.2 g of the product was obtained with a yield of 78%.
[0093] product of 1 H-NMR (400MHz, CDCl 3 ): 2.92 (bs, 1H, OH), 3.60-3.70 (m, 2H, NCH2), 3.70-3.80 (m, 2H, ClCH2), 4.16-4.19 (m, 1H, OCH), 6.48-6.50 (te, 1H, Fu-4-H), 6.79 (d, 1H, Fu-3-H), 7.53 (d, 1H, Fu-5-H), 8.14 (S, 1H, N=CH), as (S) -1-Chloro-3-[(furan-2-methylene)amino]propan-2...
Embodiment 3
[0094] Embodiment 3: Preparation of (S)-1-chloro-3-[(furan-2-methylene)amino]propan-2-ol (structural formula II)
[0095]
[0096]In the 500ml reaction bottle, add 48.0g furfural, 1160ml 95% ethanol and 45.7g ammoniacal liquor (28.8%), stir 10 minutes, add S-epichlorohydrin 46.3g, reaction exotherm, stir 1 hour, temperature rises automatically About 40°C. Heated, kept at 35-40°C, reacted for 6 hours, stirred at room temperature for 13.5 hours, concentrated to about 50ml, cooled to crystallize, filtered out the solid, and dried to obtain 47.8g of the product, with a yield of 48%.
[0097] product of 1 H-NMR (400MHz, CDCl 3 ) 2.92 (bs, 1H, OH), 3.60-3.70 (m, 2H, NCH2), 3.70-3.80 (m, 2H, ClCH2), 4.16-4.19 (m, 1H, OCH), 6.48-6.50 (te, 1H , Fu-4-H), 6.79 (d, 1H, Fu-3-H), 7.53 (d, 1H, Fu-5-H), 8.14 (S, 1H, N=CH), as (S)- 1-Chloro-3-[(furan-2-methylene)amino]propan-2-ol (Formula II).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com