Conjugation interrupted hyperbranched polymer semiconductor optoelectronic material and its preparation and application method
A technology of hyperbranched polymers and optoelectronic materials, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc. The effect of promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of 2-thiophene fluorenone
[0043] In a 100ml two-necked flask, add 2-bromofluorenone (0.9g, 0.005mol, 1eq.), thiophene boronic acid (5.00g, 38.8mmol), triphenylphosphopalladium (0.50g, 5%mmol), tetrahydrofuran / toluene (50ml), in sealing, dark, anaerobic, reaction temperature is 95 degrees centigrade, under the condition of nitrogen protection, react 30 minutes; Then inject potassium carbonate / potassium fluoride (39ml, 78mmol), continue to react for 48 hours. What needs to be added is THF / toluene, potassium carbonate / potassium fluoride are all deoxygenated solutions, and the concentration is 2mol / L. After the reaction was completed, pour ice water to quench, filter, extract the filtrate, combine the organic layers, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was purified by column with 4:1 petroleum ether / dichloromethane and dried to obtain a fluffy yellow solid 2-thiophene fluorenon...
Embodiment 2
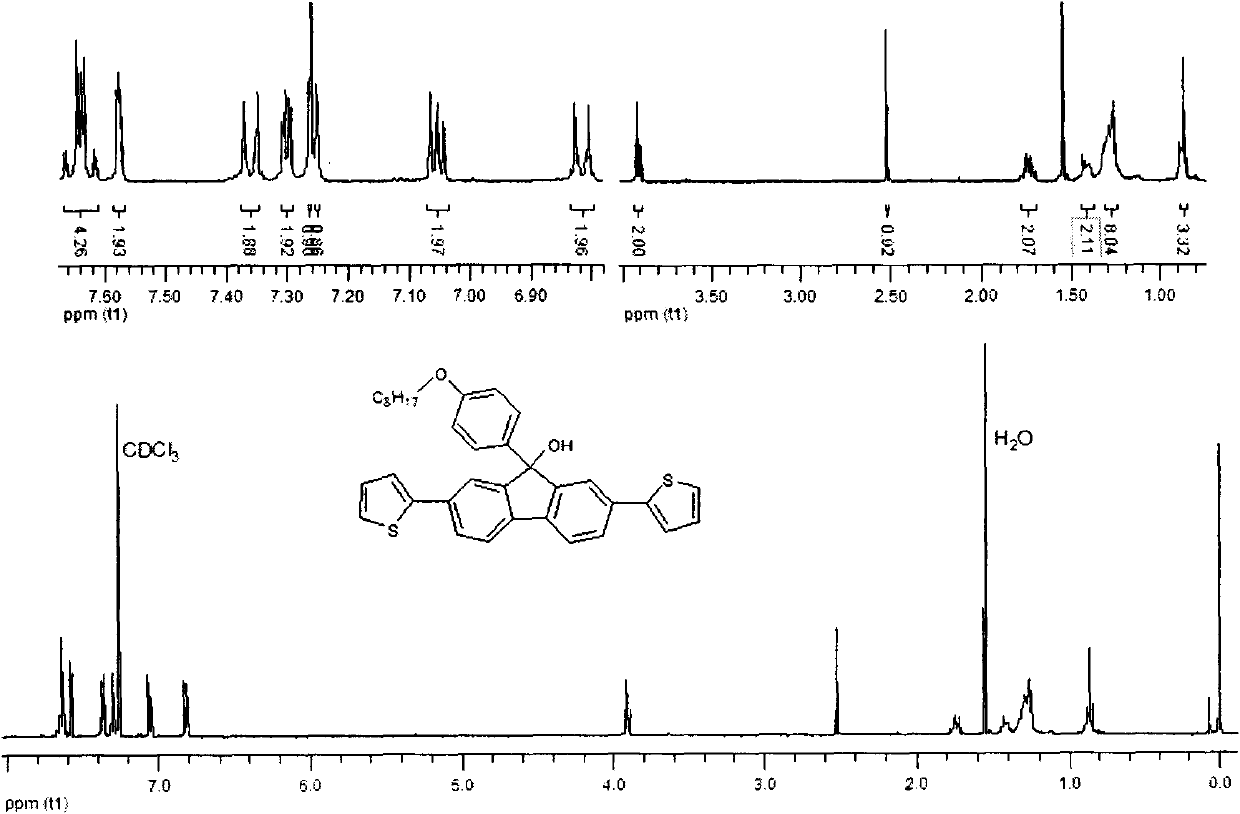
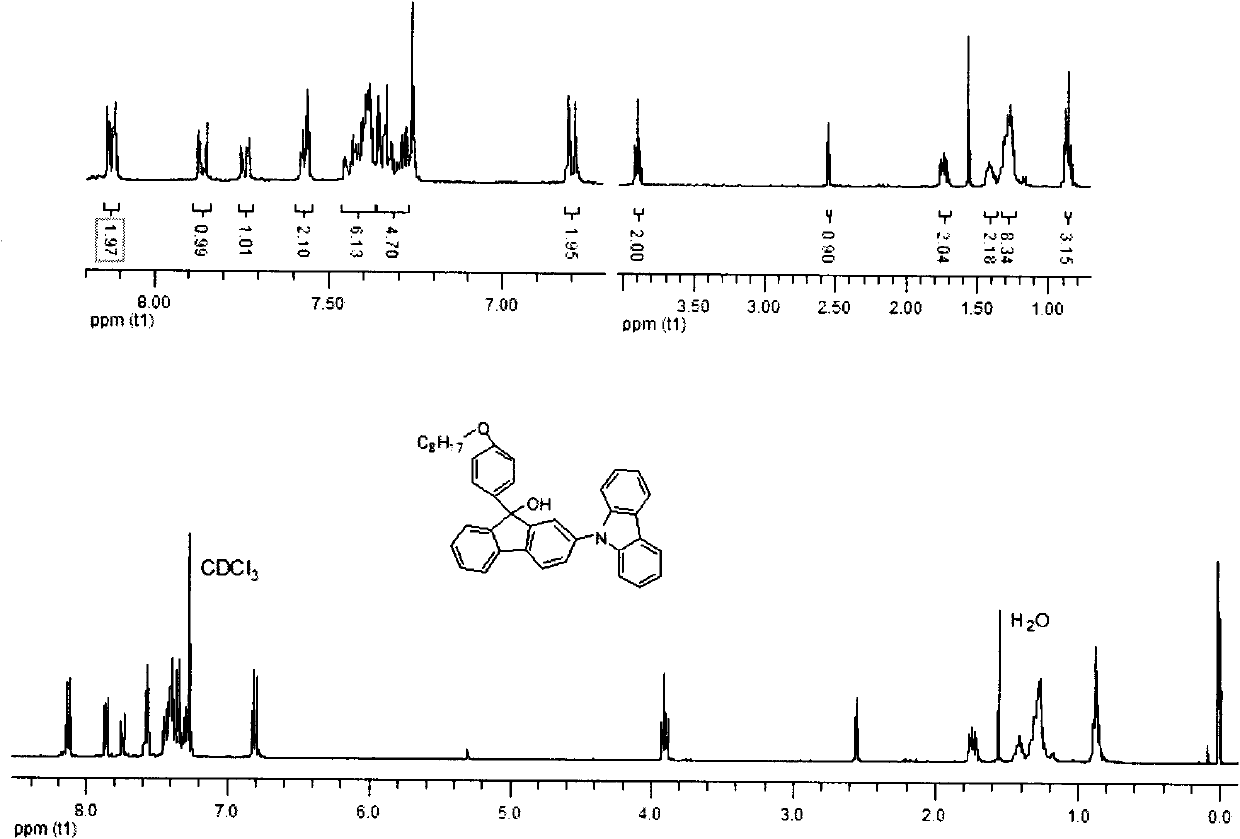
[0045] Embodiment 2: Synthesis of 2,7-dicarbazole fluorenone
[0046] In a 250ml two-necked flask, add 2,7-dibromofluorenone (6.72g, 20mmol), carbazole (8.00g, 48mmol), cuprous iodide (9.12g, 48mmol), potassium carbonate (16.60g, 120mmol) , 2,2'-bipyridine (0.005g), 1,10-phenanthroline (0.005g), 1,2-dichlorobenzene (2ml), in a sealed, dark, anaerobic, reaction temperature is 180 degrees Celsius , and reacted for 24 hours under the protection of nitrogen. After the reaction, add dichloromethane solution to quench, filter, extract the filtrate, combine organic layers, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was purified and dried by column with 6:1 petroleum ether and dichloromethane to obtain a light yellow solid 2,7-dicarbazole fluorenone. The isolated yield is 57%, and the infrared, nuclear magnetic and mass spectrometry data of this compound are as follows:
[0047] IR (KBr) v 3055, 2958, 2848, 1720, 15...
Embodiment 3
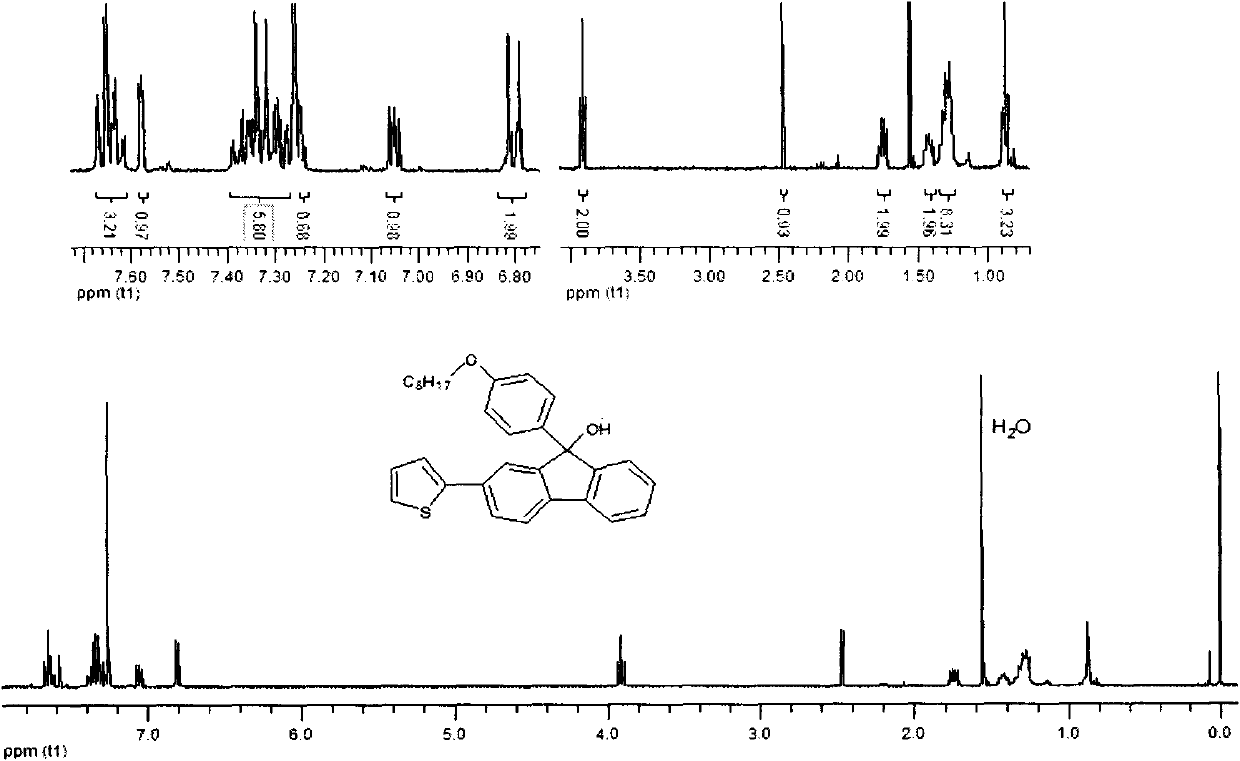
[0048] Example 3: Synthesis of p-bromooctyloxybenzene-2,7-(dithiophen-2-yl)-9 hydrogen-fluorenol
[0049] In a 250ml three-necked flask, 2,7-dithiophene fluorenone (1.12g, 3.3mmol) dissolved in 50ml of dichloromethane was slowly added dropwise to the Grignard reagent p-bromomagnesium octyloxybenzene through a constant pressure dropping funnel. (2.65g, 9.3mmol), reacted for 18 hours under the conditions of sealing, reaction temperature of 55 degrees centigrade and nitrogen protection. After the reaction was completed, add saturated ammonium chloride solution to quench, filter, extract the filtrate, combine the organic layers, anhydrous MgSO 4 Drying, rotary evaporation to remove the solvent to obtain the crude product, column purification and drying with 2:1 petroleum ether / dichloromethane to obtain white solid 9-p-bromooctyloxybenzene-2,7-(thiophen-2-yl)- 9 Hydrogen-fluorenol. The isolated yield is 79%, and the infrared, nuclear magnetic and mass spectrometry data of this co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com