A kind of isosorbide mononitrate controlled release preparation and preparation method thereof
A technology of isosorbide dinitrate and controlled-release preparations, which is applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of drug resistance and curative effect reduction, short-term, etc., and achieve the prevention of drug resistance, Improvement of treatment compliance and improvement of treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] a. Drug-containing layer prescription
[0061] name 1 tablet (mg) isosorbide mononitrate 50 povidone k90 20 lactose 30 silica 1 Magnesium stearate 1
[0062] b. Booster layer prescription
[0063] name 1 tablet (mg) Sodium carboxymethyl starch 72 hypromellose 20 carbomer 10 Sodium chloride 45 Copovidone S630 30
[0064] Iron Oxide Red 1.5 Magnesium stearate 1.5
[0065] c. Isolation gown prescription
[0066] HPMCE590% ethanol solution
[0067] d. Prescription of semi-permeable coating
[0068] Cellulose acetate: polyethylene glycol 400 (3:1) acetone solution
[0069] e. Moisture-proof gown prescription
[0070] name Dosage Opadry 5 water 40mL
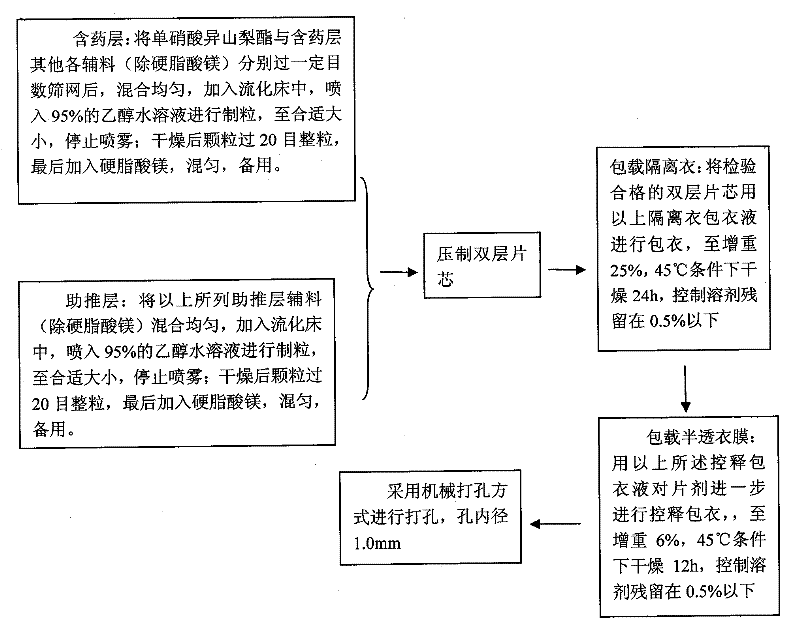
[0071] Preparation process: see Figure 8
[0072] Release measurement method:
[0073] Get this product, according to release assay (Chinese Pharmacopo...
Embodiment 2
[0075] a. Drug-containing layer prescription
[0076] name 1 tablet (mg) isosorbide mononitrate 50
[0077] povidone k90 60 EC 40 lactose 70 silica 2 Magnesium stearate 1
[0078] b. Booster layer prescription
[0079] name 1 tablet (mg) sodium alginate 66 hypromellose 20 carbomer 10 Sodium chloride 10 Copovidone S630 30 iron oxide black 1.5 Magnesium stearate 1.5
[0080] c. Isolation gown prescription
[0081] HPMCE590% ethanol solution
[0082] d. Prescription of semi-permeable coating
[0083] Cellulose acetate: povidone k30 (5:2) acetone solution
[0084] e. Moisture-proof gown prescription
[0085] name Dosage Opadry 5 water 40mL
[0086] Preparation process: see Figure 9
[0087] Release measurement method: with embodiment 1, release curve sees attached figure 2 .
Embodiment 3
[0089] a Drug-containing layer prescription
[0090] name 1 tablet (mg) isosorbide mononitrate 50 povidone k90 80 lactose 145 talcum powder 20 Magnesium stearate 5
[0091] [0091] b. Booster layer prescription
[0092] name 1 tablet (mg) Sodium carboxymethyl starch 50 carbomer 20 Mannitol 120 Iron Oxide Red 6 Magnesium stearate 4
[0093] c. Isolation gown prescription
[0094] HPMCE590% ethanol solution
[0095] d. Prescription of semi-permeable coating
[0096] Cellulose acetate: copovidone S630 (5:2) acetone solution
[0097] Preparation method: see Figure 10
[0098] Release measurement method: with embodiment 1, release curve sees attached image 3
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com