Anti-inflammatory pharmaceutical composition comprising extracts from mulberry and honeysuckle
A technology for extracts and compositions, applied in the field of anti-inflammatory pharmaceutical compositions comprising extracts from paper mulberry and honeysuckle, can solve problems such as side effects, inability to fundamentally treat COPD, and achieve less side effects or adverse effects, wide-ranging Effectiveness of therapeutic range and synergy, good anti-inflammatory and analgesic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 prepares extract by mulberry and honeysuckle
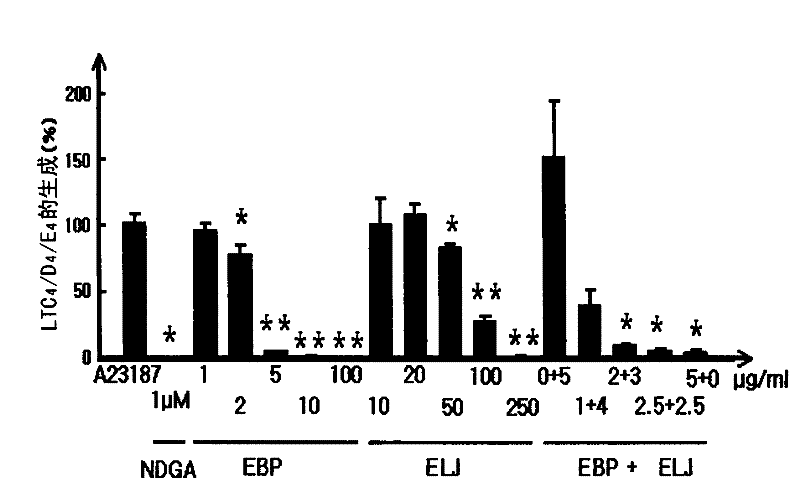
[0042] Paper mulberry is collected from the southern region of Korea (near Andong). The root bark of the mulberry tree was dried, and the dried root bark was finely cut and subjected to an extraction process using 100% ethanol. The ethanol extract was dried under vacuum and the final extract was dispersed in water and fractionated with ethyl acetate, then the ethyl acetate fraction was dried. The dried ethyl acetate fraction (EBP) was used for experiments. Papyriflavonol A and schalcone A were isolated from EBP according to the method described in Son et al. (2001).
[0043] Honeysuckle was obtained from the oriental medicine market. The dried honeysuckle was finely chopped and then subjected to an extraction process using 70% ethanol. The ethanol extract was dried under vacuum and the dried ethanol extract (ELJ) was used in the experiment. Loganin and Sweroside were isolated from this ethanol extract acco...
Embodiment 2
[0046] Example 2 Rat Basophilic Leukemia-1 (RBL-1) Cell Culture and Leukotriene (LT) Measurement
[0047] RBL-1 cells obtained from the American Type Culture Collection (American Type Culture Collection) (ATCC, Rocksville) were grown in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, and 1% antibiotics at 5% CO 2 and incubated at 37°C. Cells were placed in 96-well plates for 2 hours and pre-incubated with test substances for 10 minutes. Test substances were dissolved in DMSO and diluted with appropriate concentrations of serum-free DMEM. The final concentration of DMSO was adjusted to 0.1% (v / v). Cell viability was verified by MTT assay according to the method published in Mossman (1983). To activate 5-LOX, A-23187 (ionophore, 3 μM) was added to the cells and the cells were incubated for 15 minutes according to a slightly modified method disclosed in Tries et al. (2002). The used medium was collected and the concentration of cysteinyl leukotrienes (LTC4 / D4 / E4) produce...
Embodiment 3
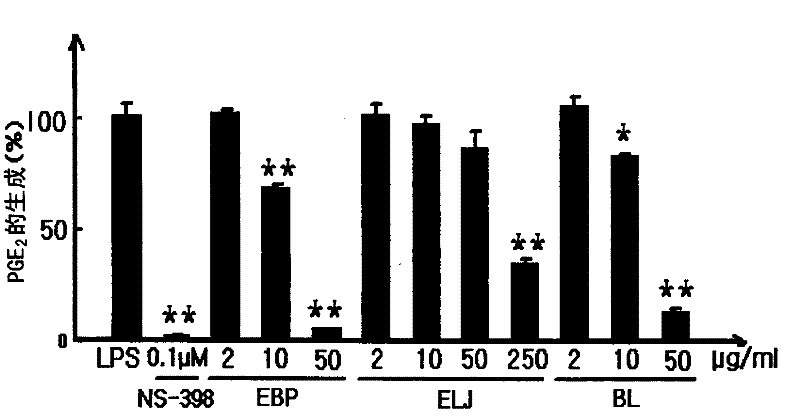
[0055] Example 3 produces PGE in RAW 264.7 cells 2 Impact
[0056] This experiment was performed to determine the effect of extracts from mulberry and honeysuckle on the production of PGE 2 Effect of PGE 2 mediator of inflammatory reactions.
[0057] RAW 264.7 cells obtained from the American Type Culture Collection (ATCC, Rocksville) were supplemented with 10% FBS and 1% antibiotics (100 U / ml penicillin and 100 μg / ml streptomycin )) in DMEM at 5% CO 2 and incubated at 37°C. The cells were activated with lipopolysaccharide (LPS) according to the method disclosed in Chi et al. (2001). Cells were loaded into 96-well plates (2x10 5 cells / well) and pre-incubated for 2 hours, then the test substance and LPS (1 μg / ml) were added thereto, and the resulting medium was incubated for 24 hours, unless otherwise specified. The concentration of PGE2 in the medium was measured by using a PGE2 ELISA kit (Cayman Chem. Co.) according to the method suggested by the manufacturer.
[0058...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com