lomefloxacin aspartate compound
A technology of lomefloxacin aspartate and aspartate, applied in organic chemistry, organic active ingredients, antibacterial drugs, etc., can solve the problems of inconvenient use, storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 30 grams of lomefloxacin and 180 ml of dimethylformamide into a 1000ml reaction bottle equipped with stirring, thermometer and condenser, start stirring, heat up to 50°C-55°C, filter, and cool the filtrate to 5°C-10°C ℃, add aspartic acid ethyl ether to pH 1-3, then cool to 0℃-5℃, keep stirring for 13 hours. The precipitated crystals were filtered, washed with 50ml of anhydrous ether in equal parts for three times, placed indoors for 1 hour, then moved to a vacuum drying oven, and dried in vacuum at room temperature for 3 hours to obtain 26.1 grams of lomefloxacin aspartate white crystalline Powder, melting point is 262-265°C, purity 99.91% (HPLC normalization method).
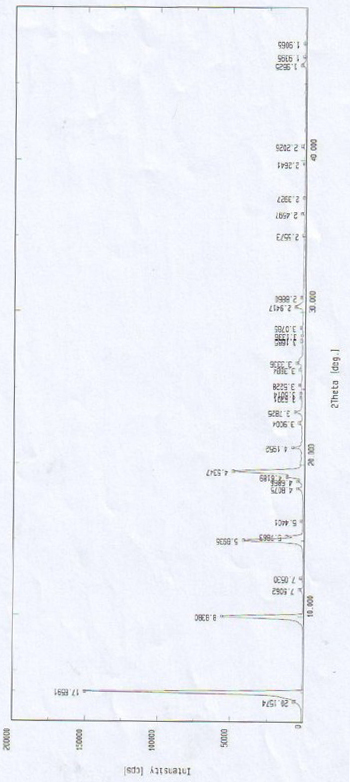
[0048] The X-ray diffraction pattern of this crystalline powder is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° ;
Embodiment 2
[0050] Granules containing a new crystal form of lomefloxacin aspartate
[0051] Prescription: 100 grams of new crystal form of lomefloxacin aspartate, 650 grams of lactose, 30 grams of microcrystalline cellulose, 68 grams of crospovidone, 80 grams of PEG-4000, 110 grams of hydroxypropyl methylcellulose grams, appropriate amount of distilled water, made into 1000 bags.
[0052] Process: PEG-4000 and the new crystal form of lomefloxacin aspartate are crushed together, passed through an 80-mesh sieve, mixed with other materials, made into soft materials with distilled water, granulated, dried at low temperature, and then packed into granules.
Embodiment 3
[0054] Capsules containing a new crystalline form of lomefloxacin aspartate
[0055] Prescription: 100 grams of new crystal form lomefloxacin aspartate, 16 ml of propylene glycol, 120 grams of starch, 26 grams of magnesium stearate, 35 grams of colloidal silicon dioxide, made into 1000 capsules.
[0056] Process: Moisten the new crystal form of lomefloxacin aspartate, starch, magnesium stearate, and colloidal silicon dioxide with 15% propylene glycol aqueous solution, mix well, sieve and granulate, dry at 60°C, and prepare Capsules, filled capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com